Posted on 10/18/2021 7:48:32 AM PDT by Red Badger

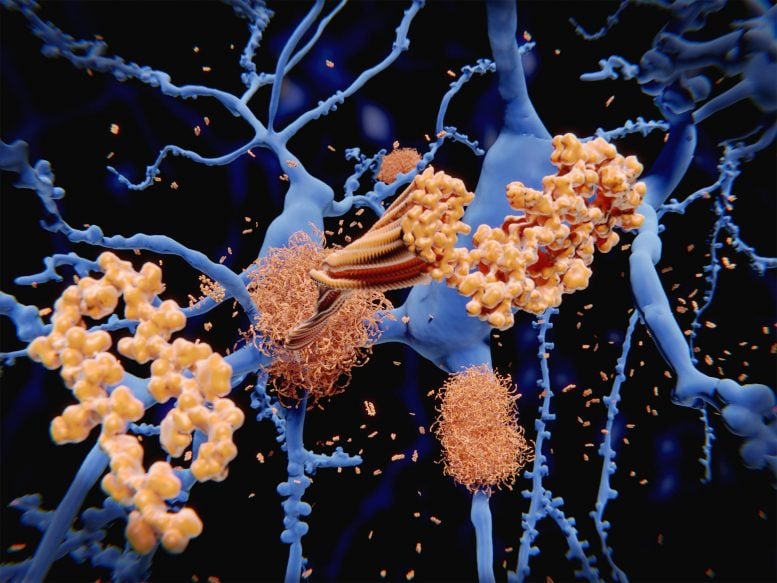
Amyloid protein (orange) forms clumps among neurons (blue). Amyloid in the brain is one of the proteins associated with Alzheimer’s disease.
================================================================================
In a major breakthrough, researchers at Massachusetts General Hospital (MGH) have discovered how amyloid beta — the neurotoxin believed to be at the root of Alzheimer’s disease (AD) — forms in axons and related structures that connect neurons in the brain, where it causes the most damage. Their findings, published in Cell Reports, could serve as a guidepost for developing new therapies to prevent the onset of this devastating neurological disease.
Among his many contributions to research on AD, Rudolph Tanzi, PhD, vice chair of Neurology and co-director of the McCance Center for Brain Health at MGH, led a team in 1986 that discovered the first Alzheimer’s disease gene, known as APP, which provides instructions for making amyloid protein precursor (APP). When this protein is cut (or cleaved) by enzymes — first, beta secretase, followed by gamma secretase — the byproduct is amyloid beta (sometimes shortened to Abeta). Large deposits of amyloid beta are believed to cause neurological destruction that results in AD. Amyloid beta formed in the brain’s axons and nerve endings causes the worst damage in AD by impairing communication between nerve cells (or neurons) in the brain. Researchers around the world have worked intensely to find ways to block the formation of amyloid beta by preventing cleavage by beta secretase and gamma secretase. However, these approaches have been hampered by safety issues.
Despite years of research, a major mystery has remained. “We knew that Abeta is made in the axons of the brain’s nerve cells, but we didn’t know how,” says Tanzi. He and his colleagues probed the question by studying the brains of mice, as well as with a research tool known as Alzheimer’s in a dish, a three-dimensional cell culture model of the disease created in 2014 by Tanzi and a colleague, Doo Yeon Kim, PhD. Earlier, in 2013, several other MGH researchers, including neurobiologist Dora Kovacs, PhD (who is married to Tanzi), and Raja Bhattacharyya, PhD, a member of Tanzi’s lab, showed that a form of APP that has undergone a process called palmitoylation (palAPP) gives rise to amyloid beta. That study indicated that, within the neuron, palAPP is transported in a fatty vesicle (or sac) known as a lipid raft. But there are many forms of lipid rafts. “So the question was, Which lipid rafts? And which ones are most relevant to the neuronal processes making up the neural networks of the brain?” says Tanzi.
The new investigation revealed that palAPP is stabilized and prepared for cleavage by beta secretase in special lipid rafts within the neuron known as mitochondria-associated endoplasmic reticulum membranes (MAMs). “We showed for the first time not only that the MAM is where palAPP is processed by beta secretase to make Abeta, but that this happens exclusively in axons and neuronal processes where Abeta does most of its damage,” says Bhattacharyya, lead author of the Cell Reports paper. This role for MAMs was previously unknown, though earlier research indicated that they are increased in number and activity in the brains of people with Alzheimer’s disease.
Next, the MGH team wanted to learn what happens when MAM levels and activity were intentionally altered. They showed for the first time that preventing assembly of MAMs, either with gene therapy or a drug that blocked a key protein called the sigma-1 receptor (S1R), dramatically decreased beta secretase cleavage of palAPP in axons and lowered Abeta production. Conversely, a drug that activated S1R triggered an increase in beta secretase cleavage of palAPP and increased production of amyloid beta in axons.
“Our results suggest that the sigma-1 receptor might be a viable therapeutic target for reducing Abeta production, specifically in axons,” says Tanzi. The study also lends support for a strategy already under investigation by Tanzi and his team, which is developing an experimental treatment that inhibits the palmitoylation of APP, the process that produces palAPP. It’s also known that another class of drugs that Kovacs is studying for preventing formation of amyloid beta, called ACAT inhibitors, works directly in MAMs. In the future, these and other interventions that thwart production of this most dangerous pool of axonal amyloid beta could be used in concert with early detection (through blood or imaging tests) to stop or slow the progression of AD.
Reference: “Axonal generation of amyloid-β from palmitoylated APP in mitochondria-associated endoplasmic reticulum membranes” by Raja Bhattacharyya, Sophia E. Black, Madhura S. Lotlikar, Rebecca H. Fenn, Mehdi Jorfi, Dora M. Kovacs and Rudolph E. Tanzi, 18 May 2021, Cell Reports. DOI: 10.1016/j.celrep.2021.109134
Tanzi directs the Genetics and Aging Research Unit and co-directs the Henry and Allison McCance Center for Brain Health at MGH and is the Joseph P. and Rose F. Kennedy Professor of Neurology at Harvard Medical School (HMS). Bhattacharyya is also an instructor in Neurology at HMS.
This study was funded by grants from the National Institutes of Health and the Cure Alzheimer’s Fund.
PING !
L.J.: enjoy your calming golden milk tonight before bed!
(The book I mentioned is good!)
Fish oil, Olive oil, some coconut oil. (No Rapeseed or Canola oil, hydrogenated fats, anything solid at room temperature! )
COCONUT OIL!
Trust me on this- take a spoonful in the morning - it takes a little getting used to, it’s just so bland.. but after a while you get used to it.
Instant energy 15 minutes later.
Prevent what? I’veforgotten already. Is it lunch time? I’d like a pizza with the works, hot peppers and a pitcher of beer.
Hmm, butter is good though, and ghee. I just can’t stand oils.
Thanks. Turmeric is a medicine cabinet in a jar.
One cannot have too much of it. I’ll up my intake. After the cancer surgery I was taking 1 heaping teaspoon a day. Can start up again. Don’t want to get stupider than I am already.
Who’d a thunk it
COCONUT OIL!
Trust me on this- take a spoonful in the morning - it takes a little getting used to, it’s just so bland.. but after a while you get used to it.
Mixing it woth rice would be more pleasant.
with
Probably ought to quit hanging out on the Qtard thread then.
Eat crappy food (old people love buffets ) and live long enough your brain turns to crap. Well I’m biased.
Coconut oil. 1/2 of its fatty acids do not need to be broken down by the body and can be directly used by your body.
That image looks like a Dali painting.
Or a Jackson Pollock one.........................
There are many theories, by non-scientists, about the “cause” of Alzheimers. Besides age or genetics, what other factors trigger the process identified by these brilliant researchers? Sugar, bad fatty diet, concussions, modern American education?
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.