Posted on 05/10/2024 11:08:14 AM PDT by Red Badger
Harnessing these molecules can significantly impact agriculture, pharmaceuticals, and electronics. Chemists at the University of Minnesota Twin Cities College of Science and Engineering have successfully synthesized a highly reactive chemical compound that has eluded sicentists for over 120 years. This breakthrough may pave the way for the development of innovative drug treatments, safer agricultural products, and enhanced electronics.
For decades, researchers have been investigating molecules called N-heteroarenes, which are ring-shaped chemical compounds that contain one or more nitrogen atoms. Bio-active molecules having a N-heteroarene core are widely used for numerous medicinal applications, lifesaving pharmaceuticals, pesticides and herbicides, and even electronics.
“While the average person does not think about heterocycles on a daily basis, these unique nitrogen-containing molecules are widely applied across all facets of human life,” said Courtney Roberts, the senior author of the study and a University of Minnesota Department of Chemistry assistant professor who holds the 3M Alumni Professorship.
Challenges in Chemical Synthesis These molecules are highly sought out by many industries, but are extremely challenging for chemists to make. Previous strategies have been able to target these specific molecules, but scientists have not been able to create a series of these molecules. One reason for this is that these molecules are extremely reactive. They are so active that chemists have used computational modeling to predict that they should be impossible to make. This has created challenges for more than a century and prevented a solution to create this chemical substance.
“What we were able to do was to run these chemical reactions with specialized equipment while getting rid of elements commonly found in our atmosphere,” said Jenna Humke, a University of Minnesota chemistry graduate student and lead author on the paper. “Luckily, we have the tools to do that at the University of Minnesota. We ran experiments under nitrogen in a closed-chamber glovebox, which creates a chemically inactive environment to test and move samples.”

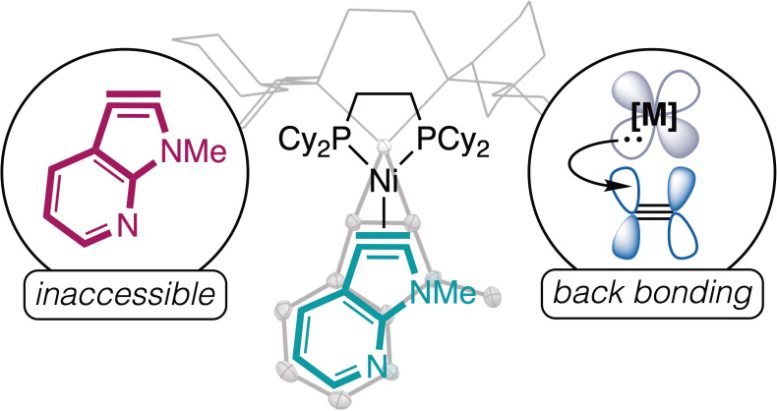
This graphic depicts the chemical compound that the team of chemists was able to discover. Credit: The Roberts Group/University of Minnesota
These experiments were accomplished by using organometallic catalysis—the interaction between metals and organic molecules. The research required collaboration between both organic and inorganic chemists. This is something that is common at the University of Minnesota.
“We were able to solve this long-standing challenge because the University of Minnesota Department of Chemistry is unique in that we don’t have formal divisions,” Roberts added. “This allows us to put together a team of experts in all fields of chemistry, which was a vital component in completing this project”
After introducing the chemical compound in this paper, the next steps will be to make it widely available to chemists across multiple fields to streamline the creation process. This could help solve important problems like preventing food scarcity and treating illnesses to save lives.
Reference: “Nickel binding enables isolation and reactivity of previously inaccessible 7-aza-2,3-indolynes” by Jenna N. Humke, Roman G. Belli, Erin E. Plasek, Sallu S. Kargbo, Annabel Q. Ansel and Courtney C. Roberts, 25 April 2024, Science. DOI: 10.1126/science.adi1606
Along with Roberts and Humke, the University of Minnesota research team included postdoctoral researcher Roman Belli, graduate students Erin Plasek, Sallu S. Kargbo, and former postdoctoral researcher Annabel Ansel.
This work was primarily funded by the National Institutes of Health and the National Science Foundation. Funding was also provided by four University of Minnesota-sponsored graduate research fellowships and start-up funding provided by the Department of Chemistry.
Please forive my ignorant utilitarianism, but what is it for?
WHY does this matter to me?
(I am not a chemist, nor do I play one on TeeVee.)
Not only that, but the average person doesn't think about heterocycles during his or her entire lifetime.
But the alphabet people DO think about homocycles 24x7.
The reason for which can be expressed in two letters: 3M. This is why retaining American manufacturing yields new science.
Harnessing these molecules can significantly impact agriculture, pharmaceuticals, and electronics.
This information is probably already on its way to China.
“Harnessing these molecules can significantly impact agriculture, pharmaceuticals, and electronics.”
Personally, that along with the rest of the self-promoting article, tells me next to nothing about what the thing is good for.
The article also says it “could help solve important problems like preventing food scarcity and treating illnesses to save lives.”
That helps, doesn’t it?
That is all well & good, but how exactly do they impact them? One would assume it’s a positive impact, but how does being able to create this as a synesthetic benefit. Especially if it occurs naturally.
N-heteroarenes are versatile molecules with diverse applications across various fields. Here's a breakdown of their specific uses:
Medicinal Applications:
Lifesaving pharmaceuticals: Many N-heteroarenes form the core structure of essential drugs, including:
Antibiotics: Ciprofloxacin, Metronidazole
Antivirals: Acyclovir, Tamiflu
Antifungal: Fluconazole
Antidepressants: Sertraline, Fluoxetine
Anti-cancer drugs: Imatinib, Gefitinib
Bioactive molecules: N-heteroarenes play a crucial role in various biological processes, including:
DNA and RNA synthesis: Adenine, Guanine, Cytosine
Neurotransmission: Dopamine, Serotonin
Cellular respiration: NADH, FADH2
Pesticides and Herbicides:
Herbicides: Atrazine, Diuron
Insecticides: Imidacloprid, Thiamethoxam
Fungicides: Triazole fungicides
Electronics:
Organic semiconductors:
N-heteroarenes are used in organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs).
Conductive polymers: N-heteroarenes are incorporated into conductive polymers for applications in batteries and solar cells.
Other Applications:
Dyes and pigments: Many N-heteroarenes are used as dyes and pigments for textiles, plastics, and paints.
Fragrances and flavors: N-heteroarenes contribute to the aroma and taste of various products.
Examples of N-Heteroarenes:
Pyridine: Used in pharmaceuticals, herbicides, and insecticides. Imidazole: Found in pharmaceuticals, fungicides, and dyes.
Pyrimidine: Essential component of DNA and RNA.
Indole: Found in the amino acid tryptophan and the neurotransmitter serotonin.
Purine: Essential component of DNA and RNA.
Conclusion: N-heteroarenes are a diverse group of molecules with a wide range of applications. Their unique properties make them valuable in various fields, including medicine, agriculture, electronics, and materials science.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.