Skip to comments.
Cosmic dust reveals Earth's ancient atmosphere
Science Daily ^
| 5/11/2016
| Monash University
Posted on 05/12/2016 10:00:37 AM PDT by JimSEA
Using the oldest fossil micrometeorites -- space dust -- ever found, Monash University-led research has made a surprising discovery about the chemistry of Earth's atmosphere 2.7 billion years ago.
The findings of a new study published today in the journal Nature -- led by Dr Andrew Tomkins and a team from the School of Earth, Atmosphere and Environment at Monash, along with scientists from the Australian Synchrotron and Imperial College, London -- challenge the accepted view that Earth's ancient atmosphere was oxygen-poor. The findings indicate instead that the ancient Earth's upper atmosphere contained about the same amount of oxygen as today, and that a methane haze layer separated this oxygen-rich upper layer from the oxygen-starved lower atmosphere.
Dr Tomkins explained how the team extracted micrometeorites from samples of ancient limestone collected in the Pilbara region in Western Australia and examined them at the Monash Centre for Electron Microscopy (MCEM) and the Australian Synchrotron.
"Using cutting-edge microscopes we found that most of the micrometeorites had once been particles of metallic iron -- common in meteorites -- that had been turned into iron oxide minerals in the upper atmosphere, indicating higher concentrations of oxygen than expected," Dr Tomkins said.
(Excerpt) Read more at sciencedaily.com ...
TOPICS: Astronomy; Science
KEYWORDS: abiogenesis; australia; biogenesis; catastrophism; earlyearth; evolution; geology; godsgravesglyphs; monashuniversity; originoflife; pilbara
Navigation: use the links below to view more comments.
first 1-20, 21-23 next last

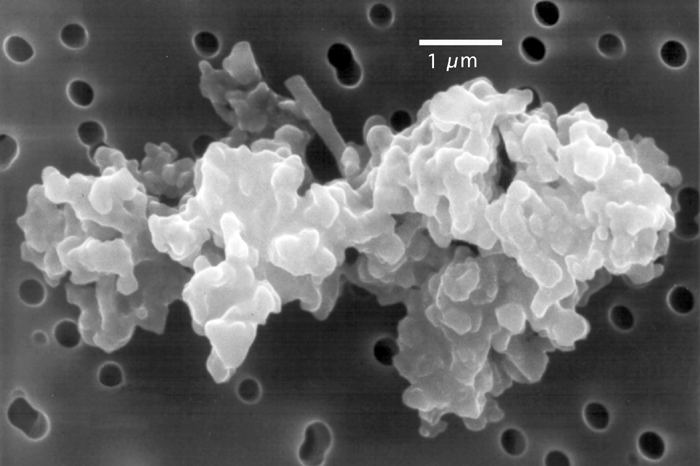
This is one of 60 micrometeorites extracted from 2.7 billion year old limestone, from the Pilbara region in Western Australia. These micrometeorites consist of iron oxide minerals that formed when dust particles of meteoritic iron metal were oxidised as they entered Earth's atmosphere, indicating that the ancient upper atmosphere was surprisingly oxygen-rich.
1
posted on
05/12/2016 10:00:37 AM PDT
by
JimSEA
To: JimSEA
Very interesting! Especially considering the theories behind the starting point where life went from anaerobic to aerobic respiration.
I wonder if the models will have to be redrawn. What caused that methane layer to dissipate? What introduced that oxygen-heavy layer, if it wasn’t CO2 respiration from new upstart life?
To: JimSEA
“Dr Tomkins explained that the new results suggest the Earth at this time may have had a layered atmosphere with little vertical mixing, and higher levels of oxygen in the upper atmosphere produced by the breakdown of CO 2 by ultraviolet light.
“A possible explanation for this layered atmosphere might have involved a methane haze layer at middle levels of the atmosphere. The methane in such a layer would absorb UV light, releasing heat and creating a warm zone in the atmosphere that would inhibit vertical mixing,” Dr Tomkins said.”
This changes a lot of what we thought we knew. It doesn’t negate earlier findings so much as it suggests more information and has implications in both abiogenesis and the search for life elsewhere in the Universe.
3
posted on
05/12/2016 10:05:49 AM PDT
by
JimSEA
To: Syncopated
4
posted on
05/12/2016 10:06:49 AM PDT
by
JimSEA
To: JimSEA
I wait with bated breath for the revelation and ensuing interpretation that tells me my use of fossil fuels, eating meat, French fries or processed foods (ooga booga) is the resulting current cause of all our ills and that I must get a wine glass and sniff my farts henceforth.
5
posted on
05/12/2016 10:15:31 AM PDT
by
Gaffer
To: JimSEA
6
posted on
05/12/2016 10:15:32 AM PDT
by
samtheman
(Trump For America.)
To: Gaffer
Well, there is nothing in this article that in any way relates to any of that.
7
posted on
05/12/2016 10:32:28 AM PDT
by
JimSEA
To: JimSEA
O2 would be heavier than CH4. So (1) what keeps it in the upper atmosphere rather than the lower; (2) why wouldn’t the methane burn at the first spark?
8
posted on
05/12/2016 10:52:32 AM PDT
by
HiTech RedNeck
(Embrace the Lion of Judah and He will roar for you and teach you to roar too. See my page.)
To: HiTech RedNeck
O2 would be heavier than CH4. So (1) what keeps it in the upper atmosphere rather than the lower; (2) why wouldn’t the methane burn at the first spark? Excellent questions.
9
posted on
05/12/2016 10:58:57 AM PDT
by
Talisker
(One who commands, must obey.)
To: HiTech RedNeck
Makes you wonder exactly what was happening at the surface that we haven’t gotten a good handle on.
10
posted on
05/12/2016 11:05:46 AM PDT
by
JimSEA
To: JimSEA
Or maybe the methane/lightning/life theory is bogus.
11
posted on
05/12/2016 11:14:46 AM PDT
by
Seruzawa
(If you agree with the French raise your hand. If you are French raise both hands)
To: Talisker; JimSEA
O2 would be heavier than CH4. So (1) what keeps it in the upper atmosphere rather than the lower; (2) why wouldn’t the methane burn at the first spark?1) Nothing "kept" the oxygen there - possibly, it was being continually formed and destroyed, i.e., released and then recombined in chemical compounds with other elements - think "ozone layer" (O3 is 50% heavier than O2, and yet the ozone layer is full of it.)
2) To burn, there would have had to have been sufficient oxygen present in the same layer as the methane; and the overall density would have had to have been sufficient (i.e.: a perfect mixture of oxygen and methane will still not combust if it is too thin).
Regards,
12
posted on
05/12/2016 11:23:52 AM PDT
by
alexander_busek
(Extraordinary claims require extraordinary evidence.)
To: JimSEA
Of course, maybe it was created to have this much oxygen in the first place.
13
posted on
05/12/2016 11:40:49 AM PDT
by
HiTech RedNeck
(Embrace the Lion of Judah and He will roar for you and teach you to roar too. See my page.)
To: alexander_busek
O3 is formed out of O2. What would you have O2 formed out of? Windborne rootless plants?
14
posted on
05/12/2016 11:42:06 AM PDT
by
HiTech RedNeck
(Embrace the Lion of Judah and He will roar for you and teach you to roar too. See my page.)
To: JimSEA
Stanley Miller, call your office.
15
posted on
05/12/2016 11:56:19 AM PDT
by
D_Idaho
("For we wrestle not against flesh and blood...")
To: alexander_busek
1. Could also just be a less dense layer of oxygen, floating over the methane. Molecular weight assumes individual interactions, charged layers could act uniformly.
2. At the boundary of oxygen and methane you could get ignition - a thin layer of fire in the sky all around the world. What a sight that would be!
16
posted on
05/12/2016 12:03:06 PM PDT
by
Talisker
(One who commands, must obey.)
To: Syncopated
Very interesting! Especially considering the theories behind the starting point where life went from anaerobic to aerobic respiration.
I wonder if the models will have to be redrawn.
...
I don’t think so. They’re still saying this is consistent with the lower atmosphere being oxygen poor.
17
posted on
05/12/2016 12:07:51 PM PDT
by
Moonman62
(Make America Great Again!)
To: Moonman62
Rock and particularly mineral formation is consistent with low oxygen at this tine as well.
18
posted on
05/12/2016 12:19:58 PM PDT
by
JimSEA
To: JimSEA
That there’s some OLD dirt!
19
posted on
05/12/2016 1:34:34 PM PDT
by
43north
(BHO: 50% black, 50% white, 100% red.)
To: JimSEA; StayAt HomeMother; Ernest_at_the_Beach; decimon; 1010RD; 21twelve; 24Karet; ...
Thanks JimSEA.

20
posted on
05/12/2016 4:10:33 PM PDT
by
SunkenCiv
(Here's to the day the forensics people scrape what's left of Putin off the ceiling of his limo.)
Navigation: use the links below to view more comments.
first 1-20, 21-23 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson