
Posted on 10/03/2009 7:46:52 AM PDT by neverdem
US scientists have confirmed the discovery of element number 114, first made over a decade ago by a team in Russia. By smashing a high energy beam of calcium-48 ions into a plutonium-242 target, the US team managed to detect two nuclei of element 114, which is predicted by some to be bordering the so-called 'island of stability' for superheavy atoms.
Yuri Oganessian and his team at Dubna, Russia, were the first to claim to have created nuclei of element 114 - but any such claim has to be thoroughly verified and the experiments repeated independently before the element can be considered for admission to the periodic table. Heino Nitsche and a team from the University of California at Berkeley spent eight days bombarding their plutonium target with calcium ions in an attempt to create the elusive element 114. 'We expected to see maybe six to eight nuclei, but we only found two!' says Nitsche. He adds that the characteristic decay sequences and energies matched very well with the Russian data for two isotopes of element 114.

|
Heino Nitsche and his team confirmed the discovery of element 114
© Roy Kaltschmidt, Berkeley
|
Like the Russians before them, Nitsche and his team used a high energy beam of 48Ca, which has eight extra neutrons compared to the most abundant isotope of calcium, 40Ca. When a 48Ca nucleus collides with a 242Pu nucleus, there is a small chance that the nuclei will fuse to create a compound nucleus with 114 protons (20 from calcium and 94 from plutonium) and 176 neutrons, explains Nitsche, 'but this is unstable and evaporates either three or four neutrons to make 287114 or 286114.'
This neutron evaporation is one of the reasons why it is difficult to confirm exactly what isotope is formed in a fusion experiment, says Nitsche, 'You know how they decay, but you can't be 100 per cent sure if the number of neutrons that evaporates is correct because you're not measuring the mass.' The fact that some of the decay products can be unknown isotopes or even unknown elements adds to the difficulty, he adds.
Oganessian says he is very happy that Nitsche's group have reproduced his team's result.
Nitsche suggests that his confirmation of the data for 114 might be enough evidence for the International Union of Pure and Applied Chemistry (Iupac) to officially recognise the Russian group as its discoverers, which would allow them to propose a name for the element, but Oganessian is stoical about the process: 'Iupac will need to check the priority and accuracy of the discovery, and that's a procedure that takes time - they have only just finished with element 112, and are still discussing the name and symbol.' He adds that it will probably not be until the end of the year that they start discussing claims to the heavier elements, including 114 and 116.
Island life
Confirmation of the existence and lifetimes of isotopes of element 114 is a big step in the hunt for a tantalising 'island of stability' predicted by theoreticians, where the lifetimes of super-heavy elements could be measured in hours, days or even millions of years. 'There are some calculations that say a nucleus with 114 protons and 184 neutrons would be stable,' says Nitsche. The two isotopes confirmed by the Berkeley group, 286114 and 287114, have half-lives of 0.13s and 0.5s respectively, but the Russian data for a heavier isotope, 289114 - made by bombarding 244Pu with 48Ca, suggest a half-life of 2.6s. 'The half-lives increase as we get closer to 184 neutrons,' says Nitsche, '2.6 seconds is an absolute eternity - people are starting to plan chemistry experiments now, to see if 114 behaves like the elements above it in the periodic table.'
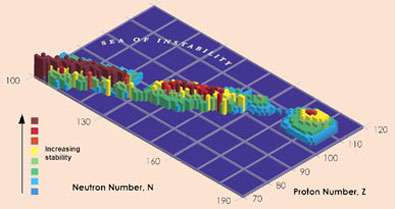
|
Scientists are tantalisingly close to the island of stability
|
But Nitsche points out that they are still at least nine neutrons away from the predicted point of stability, and to make heavier isotopes is not easy. 'Some people say the island might not be until elements 120 or 126, or there might simply be a continuous increase in stability.' He adds that experiments to make heavier elements are very important to aid theoreticians trying to understand the nucleus and make sensible predictions. Oganessian is also keen to find heavier elements, saying that his group has just begun a collaboration with scientists in the US to try and make element 117.
L Stavsetra et al, Phys. Rev. Lett., 2009, 103, 132502 (DOI: 10.1103/PhysRevLett.103.132502)

I want to understand. This is important stuff, but I’m afraid my tired old brain is so filled with air that it quickly rises to the top instead of descending into the depths of knowledge.
Um....I think I’ve had some element 114 on the bottom shelf of my refrigerator for about three years now.
Chuck Norris destroyed the periodic table, because Chuck Norris only recognizes the element of surprise.
Darned attractive for a group of scientists. The young lady in the center is particularly easy on he eye.
Yikes, is that what that gooey stuff is? My next question, is is toxic or is it worth something?
Well, I’d like to check, but I don’t want to disturb elements 1 thru 113.
This is a bit esoteric for me, but I still love it.
Thread hijack attempt.
5.56mm

^----- Composed of 99% Hottium plus trace elements
Look deeper, it hides under the bottom bin. You have to slide out the bin to search out the 114.
Cheers!
Yeah, that’s nice. Got a number for the hottie in the middle ?
Aw man! You’ve got it all! I don’t know whether i should try to become your best friend or avoid you.
http://www.youtube.com/watch?v=DYW50F42ss8
Tom Lehrer CHEMISTRY element song
governmentium:
A major research institution (MRI) has recently announced the discovery of the heaviest chemical element yet known to science. The new element has been tentatively named Governmentium. Governmentium has 1 neutron, 12 assistant neutrons, 75 deputy neutrons, and 224 assistant deputy neutrons, giving it an atomic mass of 312. These 312 particles are held together by forces called morons, which are surrounded by vast quantities of lepton-like particles called peons. Since governmentium has no electrons, it is inert. However, it can be detected as it impedes every reaction with which it comes into contact. A minute amount of governmentium causes one reaction to take over four days to complete when it would normally take less than a second. Governmentium has a normal half-life of three years; it does not decay, but instead undergoes a reorganization in which a portion of the assistant neutrons and deputy neutrons exchange places. In fact, governmentium’s mass will actually increase over time, since each reorganization will cause some morons to become neutrons, forming isodopes.
This characteristic of moron-promotion leads some scientists to speculate that governmentium is formed whenever morons reach a certain quantity in concentration. This hypothetical quantity is referred to as Critical Morass.
Will it be named “Unobtanium”?
Nope, close.
Obamaium.
Two observations:
As noted above, both young women are easy on the eyes.
Aside from that, unless they are discovering the elements Admantium or Naquadah what practical use is it?
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.