
Fourth reference to chloroquine I’ve seen today. Heard it mentioned in the presser today too. President having presser tomorrow related to the FDA. Fingers crossed.
I suppose this “commonly available anti-malaria drug known as chloroquine aka chloroquine phosphate” is made in China.
I read the Australian story yesterday saying an AIDS drug and chloroquine were effective. I hope this is the case.
This could be very good news indeed.
Re old antimalarials, wonder if anyone’s trialed some DDT ;-)
Took a pill called chloroquine-primiquine monthly in Vietnam.
All: go to the link and check out the map that says: Covid 19 is where malaria is not.
If this map is true, it is stunning.
Good to hear.
Wouldn’t quinine (tonic water) do the same thing? Had a friends who spent years in Africa who treated a relapse of malaria with quinine, while staying with us.
Zinc supplimentation plus hydroxychloroquine ... The zinc kills the virus, the chloroquine opens the virus to zinc absorption is what is being reported.
My doctor is "waiting for FDA approval process to be completed" and will not prescribe this item at present.
I am not convinced that 18 months of double-blind studies on a handful of US patients is a reasonable process. Some changes are needed here.
for later
Quinine is is tonic water. It’s difficult to find a safe way to get it if you try to do this on your own.
Potential Dangers of Homemade Tonic Water:
https://www.alcademics.com/2014/08/potential-dangers-of-homemade-tonic-water.html

This isn’t really breaking.. nor groundbreaking. Hydroxycholoroquine is frequently used for RA. However it isn’t an anti-viral and has been dismissed by many infectious disease specialists. If you’re a physician you can log into specific studies (which I cannot share unfortunately) to show that there isn’t much data to go on to give hope to this as a valid treatment.Thoughts?
MORE HERE WITH LINK TO THE SCIENTIFIC STUDIES:
http://freerepublic.com/focus/f-chat/3825971/posts
TITLE: French researcher posts successful Covid-19 drug trial that stops virus on 24 patients; US scientific researchers, replicate test with similar results
We're an independent group of scientists and physicians working on an open-data clinical trial for prevention of COVID-19, through the use of hydroxychloroquine in combination with other therapeutic agents.
Unlike a typical commercial drug trial, our objective is to share trial data with the public* and health-care professionals as close to real-time as possible (with a reasonable level of data quality assurance).
Given the rapidly spreading coronavirus pandemic, we're looking for every possible means to fast-track the effort.
* Data will be de-identified to preserve participants' privacy and conform with regulatory requirements.
Objective: Evaluate the efficacy of hydroxychloroquine in the prevention of COVID-19 infection.
Current Phase: We're first focusing on a cohort study of healthy medical professionals.
Status: Active / Recruiting
Join the study: If you're a front-line healthcare worker (physician, nurse, etc.), and willing to participate in the trial (or already taking hydroxychloroquine), please send us an email.
Future phase: Case-control study of hydroxychloroquine in the prevention of COVID-19. Stay tuned.
If you're interested to support or partner on regulatory front, clinical trial, or funding, please send us an email.
A recent well controlled clinical study conducted by Didier Raoult M.D/Ph.D, et. al in France has shown that 100% of patients that received a combination of HCQ and Azithromycin tested negative and were virologically cured within 6 days of treatment.
In addition, recent guidelines from South Korea and China report that hydroxychloroquine and chloroquine are effective antiviral therapeutic treatments for novel coronavirus.
A therapeutic agent that prevents infection with novel coronavirus is highly desirable--especially for persons with high-risk exposure (e.g healthcare professionals) as well as persons with comorbidities (heart disease, diabetes, etc) and compromised immune systems. Ground-breaking in vitro studies demonstrate potential efficacy of hydroxychloroquine as a prophylactic for novel coronavirus infection in primate cells.
Note: Hydroxychloroquine (brand name Plaquenil) is an inexpensive, globally available drug (tablet) that was approved for widespread medical use since 1955. It is commonly used today to treat malaria, systemic lupus erythematosus and rheumatoid arthritis.
Gregory J. Rigano, Esq
Mr. Rigano is an Advisor to the Stanford University School of Medicine SPARK Translational Research Program. He's led a biotech firm for the past five years in research and clinical evaluation of Chloroquine in various diseases.
Gregory has provided counsel to over $1 billion in transaction volume at global scale with a strong focus on the sciences involving multi-national corporations and the federal government. Gregory’s experience includes advancing various pharmaceutical assets through laboratory, animal, formulation, manufacturing, clinical trials (Phase I - III) as well as commercialization. Mr. Rigano received his Juris Doctor degree from Hofstra University, and studied at Johns Hopkins University.
Didier Raoult, MD & Ph.D
Didier Raoult created the Rickettsia Unit at Aix-Marseille University. Since 2008, Dr. Raoult has served as the director of URMITE (Research Unit in Infectious and Tropical Emergent Diseases), collaborating with CNRS (National Center for the Scientific Research), IRD (Research for the Development Institute), INSERM (National Institute of Health and Medical Research) and Aix Marseille University. His laboratory employs more than 200 people, including nearly 100 active researchers who publish between 250 and 350 papers per year and have produced over 50 patents.
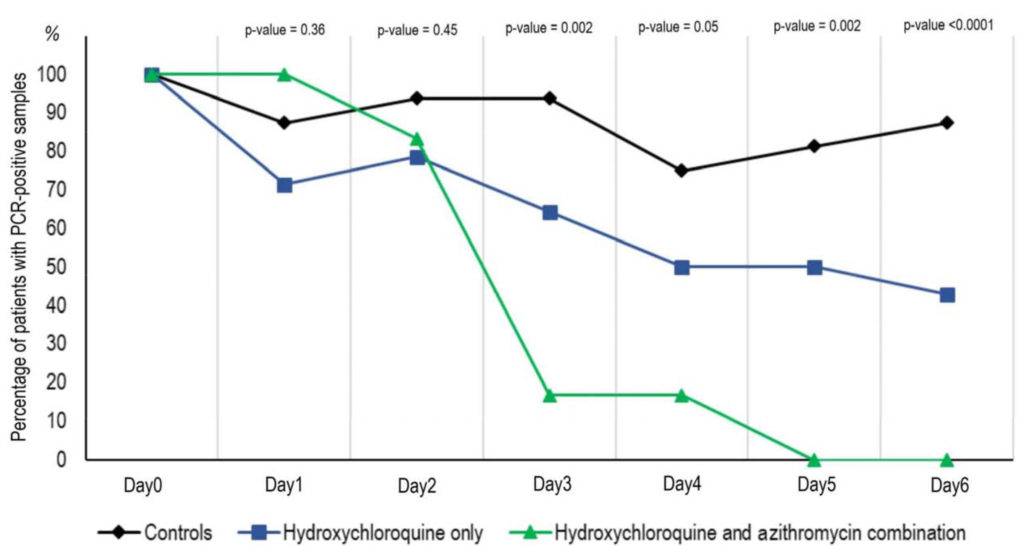
The above graph shows the Percentage of patients with PCR-positive nasopharyngeal samples from inclusion to day 6 post-inclusion in COVID-19 patients treated
with hydroxychloroquine only, in COVID-19 patients treated with hydroxychloroquine and azithomycin combination, and in COVID-19 control patients.
When the predictive number is under 0.5% for how well a study can be replicated by others, that is considered GOLDEN. If predictive number is .01%, you are 50 times better of than what is considered golden.
The said study by Dr. Raoult is .0025% in its p value ( predictive value ). This means that this drug is nearly 100% effective in killing the coronavirus (better than 99% ) and this is also the chances that any scientist who follow the same protocol will replicate the results again and again.
What does this mean?
Hydroxychloroquine are already in every pharmacy shelf in this country, so the drug will NOT BE Expensive.
They also discovered that when you mix Hydroxychloroquine with azithromycin, they have the best results on Coronavirus patients. If this is true, and studies show that it is, the goal should be to get these two drugs to as many doctors treating Covid-19 patients as possible.
That’s good news if true.