Skip to comments.
An effective treatment for #Coronavirus #COVID-19 has been found in a common anti-malarial drug
Watts up with that ^
| crazycat
Posted on 03/18/2020 7:24:32 PM PDT by crazycat
UPDATE: A Covid-19 case correlation between malarial and non-malarial countries has been plotted by Dr. Roy Spencer, and the results are stunning – see below.
Encouraging news: three new medical studies show a commonly available anti-malaria drug known as chloroquine aka chloroquine phosphate is showing strong results against COVID-19 infections in both China and South Korea. Excerpts from three studies, including one published in Nature are below.
(Excerpt) Read more at wattsupwiththat.com ...
TOPICS: Chit/Chat
KEYWORDS: braking; corona; postedseveraltimes; virus
Navigation: use the links below to view more comments.
first previous 1-20 ... 41-60, 61-80, 81-100 ... 121-122 next last
To: mmichaels1970
That would be a great way to expose them. Might open peoples minds on what the FDA actually is.
To: AFreeBird
According to Watts, the drug alters the Blood PH level, which pisses off the virus.
62
posted on
03/18/2020 8:25:03 PM PDT
by
crazycat
To: scripter
1. Hydroxychloriquine was used to treat SARS.
Other than that, I’m admittedly running in hopes and prayers.
This
link seems to contain instructions for pharmacists. So I feel like somebody somewhere, who is much smarter than me, is taking this treatment very seriously.
To: crazycat
Raises alkalinity, I presume?
To: crazycat
MORE HERE WITH LINK TO THE SCIENTIFIC STUDIES:
http://freerepublic.com/focus/f-chat/3825971/posts
TITLE: French researcher posts successful Covid-19 drug trial that stops virus on 24 patients; US scientific researchers, replicate test with similar results
65
posted on
03/18/2020 8:38:17 PM PDT
by
SeekAndFind
(look at Michigan, it will)
To: Karl Spooner
Pass...no idea....just what I read.
66
posted on
03/18/2020 8:38:55 PM PDT
by
crazycat
To: SeekAndFind
I don’t trust the Chinese as far as I can throw em. But
here is a link where they are apparently claiming it works very well. Hard to read through medical study stuff though.
To: crazycat
MEANWHILE IN THE USA ...
An Open Data Clinical Trial for COVID-19 Prevention
Clinical trial by Gautret, Raoult, et al. (2020)What is this initiative?
We're an independent group of scientists and physicians working on an open-data clinical trial for prevention of COVID-19, through the use of hydroxychloroquine in combination with other therapeutic agents.
Unlike a typical commercial drug trial, our objective is to share trial data with the public* and health-care professionals as close to real-time as possible (with a reasonable level of data quality assurance).
Given the rapidly spreading coronavirus pandemic, we're looking for every possible means to fast-track the effort.
> Read our draft paper
* Data will be de-identified to preserve participants' privacy and conform with regulatory requirements.
How can I participate in the trial?
Objective: Evaluate the efficacy of hydroxychloroquine in the prevention of COVID-19 infection.
Current Phase: We're first focusing on a cohort study of healthy medical professionals.
Status: Active / Recruiting
Join the study: If you're a front-line healthcare worker (physician, nurse, etc.), and willing to participate in the trial (or already taking hydroxychloroquine), please send us an email.
Future phase: Case-control study of hydroxychloroquine in the prevention of COVID-19. Stay tuned.
Can my company / organization participate in the trial?
We'd be happy to discuss.
Could I support the project in other ways?
If you're interested to support or partner on regulatory front, clinical trial, or funding, please send us an email.
Background
A recent well controlled clinical study conducted by Didier Raoult M.D/Ph.D, et. al in France has shown that 100% of patients that received a combination of HCQ and Azithromycin tested negative and were virologically cured within 6 days of treatment.
In addition, recent guidelines from South Korea and China report that hydroxychloroquine and chloroquine are effective antiviral therapeutic treatments for novel coronavirus.
A therapeutic agent that prevents infection with novel coronavirus is highly desirable--especially for persons with high-risk exposure (e.g healthcare professionals) as well as persons with comorbidities (heart disease, diabetes, etc) and compromised immune systems. Ground-breaking in vitro studies demonstrate potential efficacy of hydroxychloroquine as a prophylactic for novel coronavirus infection in primate cells.
Note: Hydroxychloroquine (brand name Plaquenil) is an inexpensive, globally available drug (tablet) that was approved for widespread medical use since 1955. It is commonly used today to treat malaria, systemic lupus erythematosus and rheumatoid arthritis.
Project Lead
Gregory J. Rigano, Esq
Mr. Rigano is an Advisor to the Stanford University School of Medicine SPARK Translational Research Program. He's led a biotech firm for the past five years in research and clinical evaluation of Chloroquine in various diseases.
Gregory has provided counsel to over $1 billion in transaction volume at global scale with a strong focus on the sciences involving multi-national corporations and the federal government. Gregory’s experience includes advancing various pharmaceutical assets through laboratory, animal, formulation, manufacturing, clinical trials (Phase I - III) as well as commercialization. Mr. Rigano received his Juris Doctor degree from Hofstra University, and studied at Johns Hopkins University.
Consulting Scientists & Physicians
Didier Raoult, MD & Ph.D
Didier Raoult created the Rickettsia Unit at Aix-Marseille University. Since 2008, Dr. Raoult has served as the director of URMITE (Research Unit in Infectious and Tropical Emergent Diseases), collaborating with CNRS (National Center for the Scientific Research), IRD (Research for the Development Institute), INSERM (National Institute of Health and Medical Research) and Aix Marseille University. His laboratory employs more than 200 people, including nearly 100 active researchers who publish between 250 and 350 papers per year and have produced over 50 patents.
68
posted on
03/18/2020 8:44:00 PM PDT
by
SeekAndFind
(look at Michigan, it will)
To: SeekAndFind
Tks...this needs to pushed out...as for some reason the MSM...politicians are not talking about it.
69
posted on
03/18/2020 8:45:08 PM PDT
by
crazycat
To: crazycat
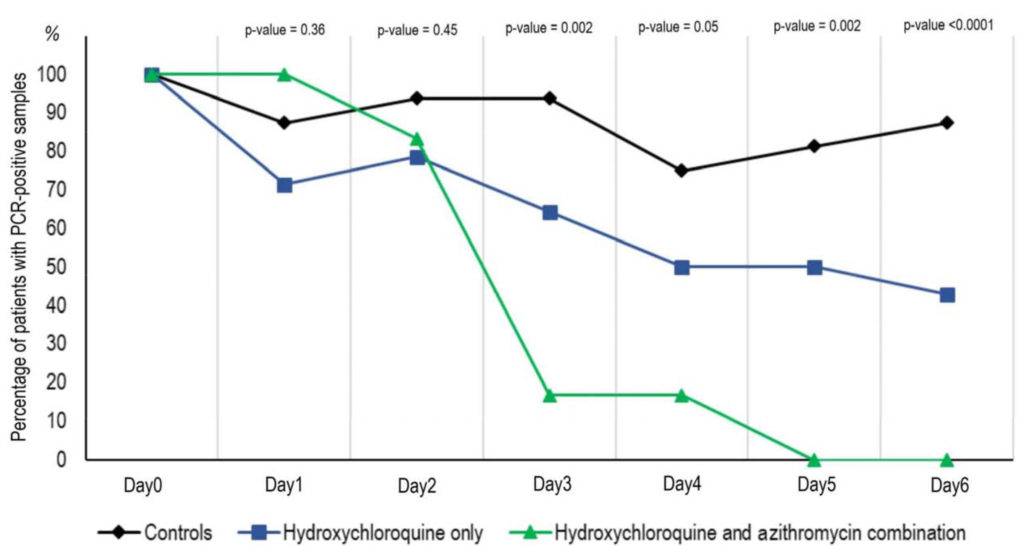
Please cite this work as Gautret et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents – In Press 17 March 2020 – DOI : 10.1016/j.ijantimicag.2020.105949
CLICK
THIS LINK for the PDF document of this study
The above graph shows the Percentage of patients with PCR-positive nasopharyngeal samples from inclusion to day 6 post-inclusion in COVID-19 patients treated
with hydroxychloroquine only, in COVID-19 patients treated with hydroxychloroquine and azithomycin combination, and in COVID-19 control patients.
70
posted on
03/18/2020 8:45:57 PM PDT
by
SeekAndFind
(look at Michigan, it will)
To: crazycat
71
posted on
03/18/2020 8:48:12 PM PDT
by
SeekAndFind
(look at Michigan, it will)
To: crazycat
72
posted on
03/18/2020 8:50:19 PM PDT
by
SeekAndFind
(look at Michigan, it will)
To: SeekAndFind
Tks...lets hope this gets traction and reaches the right people.....like the Donald.
73
posted on
03/18/2020 8:57:02 PM PDT
by
crazycat
To: crazycat
When the predictive number is under 0.5% for how well a study can be replicated by others, that is considered GOLDEN. If predictive number is .01%, you are 50 times better of than what is considered golden.
The said study by Dr. Raoult is .0025% in its p value ( predictive value ). This means that this drug is nearly 100% effective in killing the coronavirus (better than 99% ) and this is also the chances that any scientist who follow the same protocol will replicate the results again and again.
What does this mean?
Hydroxychloroquine are already in every pharmacy shelf in this country, so the drug will NOT BE Expensive.
They also discovered that when you mix Hydroxychloroquine with azithromycin, they have the best results on Coronavirus patients. If this is true, and studies show that it is, the goal should be to get these two drugs to as many doctors treating Covid-19 patients as possible.
74
posted on
03/18/2020 9:01:00 PM PDT
by
SeekAndFind
(look at Michigan, it will)
To: AFreeBird; crazycat; mmichaels1970
Thanks for the replies.
I haven’t been on FR much lately and have been on Facebook. Having said that, I don’t know what everybody’s been talking about here but I’m thinking it’s similar to what I’ve seen.
The infection and mortality rates are not nearly what we were told. From the CDC, we’re at 1.3% mortality rate, right there with the flu. Then considering the age, health, diseases, etc of the deceased, we see a specific segment of the populace are hit hardest. So, why are we nearing martial law with something like the flu?
75
posted on
03/18/2020 9:10:33 PM PDT
by
scripter
To: crazycat
That’s good news if true.
To: crazycat
77
posted on
03/18/2020 9:21:34 PM PDT
by
Pelham
(RIP California, killed by massive immigration)
To: musicman
78
posted on
03/18/2020 9:25:20 PM PDT
by
musicman
(The future is just a collection of successive nows.)
To: crazycat
Very dangerous side effects. The true answer Discovering the Universal Antimicrobial Silver is one of the most useful, versatile, safe, and effective antibiotic substances known to medical science. When administered in the colloidal form (at around 10 ppm ed.), it is for all practical purposes, non-toxic*. Silver has been proven to be useful against hundreds of infectious conditions. Although the exact mechanism for the proven antimicrobial effects of silver is unknown, the most accepted theory is that silver disables the specific enzyme that many forms of bacteria, viruses, and fungi utilize for their metabolism
79
posted on
03/18/2020 9:39:43 PM PDT
by
Truthoverpower
(The guv mint you get is the Trump winning express !)
To: flamberge
I’m pretty sure you just flew to Indonesia and contracted malaria, didn’t you?
80
posted on
03/18/2020 9:44:03 PM PDT
by
griffin
Navigation: use the links below to view more comments.
first previous 1-20 ... 41-60, 61-80, 81-100 ... 121-122 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson

