Posted on 12/29/2022 11:44:06 AM PST by Red Badger

Extreme Pressure Diamond Anvil
Structures and properties of materials at extremely high pressures and temperatures are still largely “terra incognita”. Prof. Leonid Dubrovinsky and his research partners use a laser-heated two-stage diamond anvil cell they constructed for the synthesis of materials in the terapascal range (1000 gigapascals). In situ single crystal X-ray diffraction is used for the simultaneous structural characterization of the materials. Credit: Timofey Fedotenko
******************************************************************
New method enables materials synthesis research and study in the terapascal range for the first time.
Jules Verne could not have dreamed of this: A research team from the University of Bayreuth, together with international partners including scientists from the University of Cologne’s Department of Chemistry, has pushed the boundaries of high-pressure and high-temperature research into cosmic dimensions. They succeeded in generating and simultaneously analyzing materials under compression pressures of more than one terapascal (1,000 gigapascals) for the first time. Such extremely high pressures prevail, for example, at the center of the planet Uranus; they are more than three times higher than the pressure at the center of the Earth. In the journal Nature, the researchers present the method they have developed for the synthesis and structural analysis of novel materials.
Theoretical models predict very unusual structures and properties of materials under extreme pressure-temperature conditions. But so far, these predictions could not be verified in experiments at compression pressures of more than 200 gigapascals. On the one hand, complex technical requirements are necessary to expose material samples to such extreme pressures, and on the other hand, sophisticated methods for simultaneous structural analyses were lacking. The experiments published in Nature, therefore, open up completely new dimensions for high-pressure crystallography: materials can now be created and studied in the laboratory that exist – if at all – only under extremely high pressures in the vastness of the Universe.
“The method we have developed enables us for the first time to synthesize new material structures in the terapascal range and to analyze them in situ – that is: while the experiment is still running. In this way, we learn about previously unknown states, properties, and structures of crystals and can significantly deepen our understanding of matter in general. Valuable insights can be gained for the exploration of terrestrial planets and the synthesis of functional materials used in innovative technologies,” Professor Dr. Leonid Dubrovinsky of the Bavarian Research Institute of Experimental Geochemistry and Geophysics (BGI) at the University of Bayreuth, the lead author of the publication.
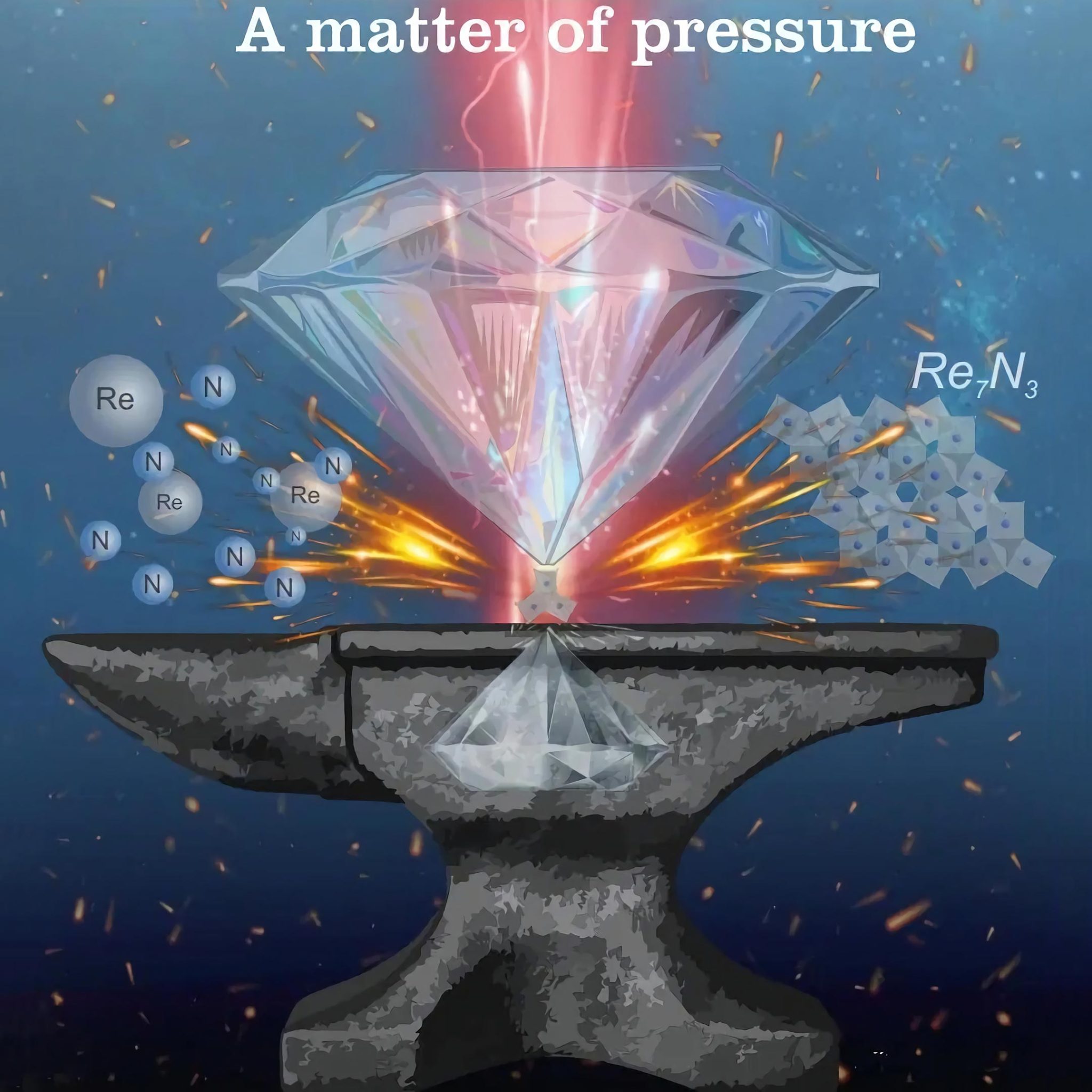
In their study, the researchers show how they have generated and visualized in situ novel rhenium compounds using the now-developed method. The compounds in question are a novel rhenium nitride (Re7N3) and a rhenium-nitrogen alloy. These materials were synthesized under extreme pressures in a two-stage diamond anvil cell heated by laser beams. Synchrotron single-crystal X-ray diffraction enabled full chemical and structural characterization.
“Rhenium-nitrogen system is full of chemical surprises. It attracted our attention several years ago, when we produced an unusual porous compound ReN10 at a pressure of one million atmospheres as well as a superhard metallic conductor ReN2 that could withstand even extremely high compression. Synthesis at one terapascal finally allowed us to get the full picture of chemical transformations, which can occur in the Re-N system at extreme conditions,” said Dr. Maxim Bykov from the Institute of Inorganic Chemistry at the University of Cologne.
“If we apply high-pressure crystallography in the terapascal range in the future, we may make further surprising discoveries in this direction. The doors are now wide open for creative materials research that generates and visualizes unexpected structures under extreme pressures,” said the study’s other lead author, Professor Dr. Natalia Dubrovinskaia from the Laboratory of Crystallography at the University of Bayreuth.
Reference:
“Materials synthesis at terapascal static pressures” by Leonid Dubrovinsky, Saiana Khandarkhaeva, Timofey Fedotenko, Dominique Laniel, Maxim Bykov, Carlotta Giacobbe, Eleanor Lawrence Bright, Pavel Sedmak, Stella Chariton, Vitali Prakapenka, Alena V. Ponomareva, Ekaterina A. Smirnova, Maxim P. Belov, Ferenc Tasnádi, Nina Shulumba, Florian Trybel, Igor A. Abrikosov and Natalia Dubrovinskaia, 11 May 2022, Nature.
DOI: 10.1038/s41586-022-04550-2
Together with the Bavarian Research Institute of Experimental Geochemistry and Geophysics (BGI) and the Laboratory of Crystallography at the University of Bayreuth, numerous other research partners were involved in the research work published in Nature: the University of Cologne, the University of Linköping, the German Electron Synchrotron DESY in Hamburg, the European Synchrotron Radiation Facility in Grenoble and the Center for Advanced Radiation Sources at the University of Chicago.
So, in a billion years or so, all the diamonds will explode?..................cool!..............
Yeah, longer than that. The diamond you dig up today will turn to graphite by the time the universe collapses into a black hole.
Diamonds may not be forever, but close enough for government work.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.