Skip to comments.
A SECOND LOOK AT THE SECOND LAW : CAN ANYTHING HAPPEN IN AN OPEN SYSTEM ?
Math Dept., Texas A&M University ^
| Granville Sewell
Posted on 10/19/2006 4:36:37 PM PDT by SirLinksalot
A Second Look at the Second Law
Granville Sewell
Mathematics Dept.
Texas A&M University
Can ANYTHING Happen in an Open System?
In the current debate over "Intelligent Design," the strongest argument offered by opponents of design is this: we have scientific explanations for most everything else in Nature, what is special about evolution? The layman understands quite well that explaining the appearance of human brains is a very different sort of problem from finding the causes of earthquakes; however, to express this difference in terms a scientist can understand requires a discussion of the second law of thermodynamics.
The first formulations of the second law were all about heat: a quantity called thermal "entropy" was defined to measure the randomness, or disorder, associated with a temperature distribution, and it was shown that in an isolated system this entropy always increases, or at least never decreases, as the temperature becomes more and more randomly (more uniformly) distributed. If we define thermal "order" to be the opposite (negative) of thermal entropy, we can say that the thermal order can never increase in a closed (isolated) system. However, it was soon realized that other types of order can be defined which also never increase in a closed system, for example, we can define a "carbon order" associated with the distribution of carbon diffusing in a solid, using the same equations, and through an identical analysis show that this order also continually decreases, in a closed system. With time, the second law came to be interpreted more and more generally, and today most discussions of the second law in physics textbooks offer examples of entropy increases (or order decreases, since we are defining order to be the opposite of entropy) which have nothing to do with heat conduction or diffusion, such as the shattering of a wine glass or the demolition of a building.
For example, in Basic Physics [Ford 1968] Kenneth Ford writes, "Imagine a motion picture of any scene of ordinary life run backward. You might watch...a pair of mangled automobiles undergoing instantaneous repair as they back apart. Or a dead rabbit rising to scamper backward into the woods as a crushed bullet re-forms and flies backward into a rifle while some gunpowder is miraculously manufactured out of hot gas. Or something as simple as a cup of coffee on a table gradually becoming warmer as it draws heat from its cooler surroundings. All of these backward-in-time views and a myriad more that you can quickly think of are ludicrous and impossible for one reason only--they violate the second law of thermodynamics. In the actual scene of events, entropy is increasing. In the time reversed view, entropy is decreasing."
It is a well-known prediction of the second law that, in a closed system, every type of order is unstable and must eventually decrease, as everything tends toward more probable states. Natural forces, such as corrosion, erosion, fire and explosions, do not create order, they destroy it. S. Angrist and L. Hepler, in Order and Chaos [Angrist and Hepler, 1967], write, "An arsonist working on a big library is merely speeding up the inevitable result demanded by the second law."
The second law is all about probability, it uses probability at the microscopic level to predict macroscopic change: the reason carbon distributes itself more and more uniformly in an insulated solid is, that is what the laws of probability predict when diffusion alone is operative. The reason natural forces may turn a spaceship, or a TV set, or a computer into a pile of rubble but not vice-versa is also probability: of all the possible arrangements atoms could take, only a very small percentage could fly to the moon and back, or receive pictures and sound from the other side of the Earth, or add, subtract, multiply and divide real numbers with high accuracy.
The discovery that life on Earth developed through evolutionary "steps," coupled with the observation that mutations and natural selection -- like other natural forces -- can cause (minor) change, is widely accepted in the scientific world as proof that natural selection -- alone among all natural forces -- can create order out of disorder, and even design human brains, with human consciousness. Only the layman seems to see the problem with this logic. In a recent Mathematical Intelligencer article [Sewell 2000], I asserted that the idea that the four fundamental forces of physics alone could rearrange the fundamental particles of Nature into spaceships, nuclear power plants, and computers, connected to laser printers, CRTs, keyboards and the Internet, appears to violate the second law of thermodynamics in a spectacular way.
Anyone who has made such an argument is familiar with the standard reply: the Earth is an open system, it receives energy from the sun, and order can increase in an open system, as long as it is "compensated" somehow by a comparable or greater decrease outside the system. S. Angrist and L. Hepler, for example (again in Order and Chaos ) write, "In a certain sense the development of civilization may appear contradictory to the second law.... Even though society can effect local reductions in entropy, the general and universal trend of entropy increase easily swamps the anomalous but important efforts of civilized man. Each localized, man-made or machine-made entropy decrease is accompanied by a greater increase in entropy of the surroundings, thereby maintaining the required increase in total entropy."
According to this reasoning, then, the second law does not prevent scrap metal from reorganizing itself into a computer in one room, as long as two computers in the next room are rusting into scrap metal -- and the door is open. (Or the thermal entropy in the next room is increasing--though I'm not sure what the conversion rate is between computers and thermal entropy.) This strange argument of "compensation" makes no sense logically: an extremely improbable event is not rendered less improbable by the occurrence of other events which are more probable. To understand where this argument of compensation comes from, one needs to understand that of the example applications mentioned in the Ford text above, the coffee cup example is special: the application to heat conduction is special not only because it was the first application, but because it is quantifiable. It is commonly used as the "model" problem on which our thinking about the other, less quantifiable, applications is based. The fact that thermal order cannot increase in a closed system, but can increase in an open system, was used to conclude that, in other applications, anything can happen in an open system as long as it is compensated by order decreases outside this system, so that the total "order" in the universe (or any closed system containing the open system) still decreases.
In Appendix D of my new book [Sewell, 2005], I take a closer look at the equations for entropy change, which apply not only to thermal entropy but also to the entropy associated with anything else that diffuses, and show that they do not simply say that order cannot increase in a closed system, they also say that in an open system, order cannot increase faster than it is imported through the boundary. According to these equations, the thermal order in an open system can decrease in two different ways -- it can be converted to disorder, or it can be exported through the boundary. It can increase in only one way: by importation through the boundary. Similarly, the increase in "carbon order" in an open system cannot be greater than the carbon order imported through the boundary, and the increase in "chromium order" cannot be greater than the chromium order imported through the boundary, and so on.
The "compensation" argument was produced by people who generalized the model equation for closed systems, but forgot to generalize the equation for open systems. Both equations are only valid for our simple models, where it is assumed that only heat conduction or diffusion is going on; naturally in more complex situations, the laws of probability do not make such simple predictions. Nevertheless, in [Sewell 2001] I generalized the equation for open systems to the following tautology, which is valid in all situations: "If an increase in order is extremely improbable when a system is closed, it is still extremely improbable when the system is open, unless something is entering which makes it not extremely improbable." The fact that order is disappearing in the next room does not make it any easier for computers to appear in our room -- unless this order is disappearing into our room, and then only if it is a type of order that makes the appearance of computers not extremely improbable, for example, computers. Importing thermal order will make the temperature distribution less random, and importing carbon order will make the carbon distribution less random, but neither makes the formation of computers more probable.
What happens in a closed system depends on the initial conditions; what happens in an open system depends on the boundary conditions as well. As I wrote in "Can ANYTHING Happen in an Open System?" [Sewell, 2001] "order can increase in an open system, not because the laws of probability are suspended when the door is open, but simply because order may walk in through the door.... If we found evidence that DNA, auto parts, computer chips, and books entered through the Earth's atmosphere at some time in the past, then perhaps the appearance of humans, cars, computers, and encyclopedias on a previously barren planet could be explained without postulating a violation of the second law here (it would have been violated somewhere else!). But if all we see entering is radiation and meteorite fragments, it seems clear that what is entering through the boundary cannot explain the increase in order observed here."
Darwin's Order Source The evolutionist, therefore, cannot avoid the question of probability by saying that anything can happen in an open system, he is finally forced to argue that it only seems extremely improbable, but really isn't, that atoms would rearrange themselves into spaceships and computers and TV sets.
Darwinists believe they have already discovered the source of all this order, so let us look more closely at their theory. The traditional argument against Darwinism is that natural selection cannot guide the development of new organs and new systems of organs -- i.e., the development of new orders, classes and phyla -- through their initial useless stages, during which they provide no selective advantage. Natural selection may be able to darken the wings of a moth (even this is disputed), but that does not mean it can design anything complex. French biologist Jean Rostand [Rostand 1956], writes "I believe firmly in the evolution of organic Nature," yet says
It does not seem strictly impossible that mutations should have introduced into the animal kingdom the differences which exist between one species and the next...hence it is very tempting to lay also at their door the differences between classes, families and orders, and, in short, the whole of evolution. But it is obvious that such an extrapolation involves the gratuitous attribution to the mutations of the past of a magnitude and power of innovation much greater than is shown by those of today.
Consider, for example, the aquatic bladderwort, described in Plants and Environment [Daubenmire 1947]:
The aquatic bladderworts are delicate herbs that bear bladder-like traps 5mm or less in diameter. These traps have trigger hairs attached to a valve-like door which normally keeps the trap tightly closed. The sides of the trap are compressed under tension, but when a small form of animal life touches one of the trigger hairs the valve opens, the bladder suddenly expands, and the animal is sucked into the trap. The door closes at once, and in about 20 minutes the trap is set ready for another victim.
In a Nature Encyclopedia of Life Sciences [Nature Publishing Group, 2004] article on Carnivorous Plants, authors Wolf-Ekkehard Lonnig and Heinz-Albert Becker acknowledge that "it appears to be hard to even imagine a clearcut selective advantage for all the thousands of postulated intermediate steps in a gradual scenario...for the origin of the complex carnivorous plant structures examined above."
The development of any major new feature presents similar problems, and according to Lehigh University biochemist Michael Behe, who describes several spectacular examples in detail in Darwin's Black Box [Behe 1996], the world of microbiology is especially loaded with such examples of "irreducible complexity."
It seems that until the trigger hair, the door, and the pressurized chamber were all in place, and the ability to digest small animals, and to reset the trap to be able to catch more than one animal, had been developed, none of the individual components of this carnivorous trap would have been of any use. What is the selective advantage of an incomplete pressurized chamber? To the casual observer, it might seem that none of the components of this trap would have been of any use whatever until the trap was almost perfect, but of course a good Darwinist will imagine two or three far-fetched intermediate useful stages, and consider the problem solved. I believe you would need to find thousands of intermediate stages before this example of irreducible complexity has been reduced to steps small enough to be bridged by single random mutations -- a lot of things have to happen behind the scenes and at the microscopic level before this trap could catch and digest animals. But I don't know how to prove this. (Lest anyone imagine a lot can be accomplished by single random mutations, note that if a billion animals each typed one random character per second throughout the Earth's 4.5 billion year history, there is virtually no chance any one of them would duplicate a given 20-character string.)
I am furthermore sure that even if you could imagine a long chain of useful intermediate stages, each would present such a negligible selective advantage that nothing as clever as this carnivorous trap could ever be produced, but I can't prove that either. Finally, that natural selection seems even remotely plausible depends on the fact that while species are awaiting further improvements, their current complex structure is "locked in," and passed on perfectly through many generations. This phenomenon is observed, but inexplicable -- I don't see any reason why all living organisms do not constantly decay into simpler components -- as, in fact, they do as soon as they die.
When you look at the individual steps in the development of life, Darwin's explanation is difficult to disprove, because some selective advantage can be imagined in almost anything. Like every other scheme designed to violate the second law, it is only when you look at the net result that it becomes obvious it won't work.
A National Geographic article from November 2004 proclaims that the evidence is "overwhelming" that Darwin was right about evolution. Since there is no proof that natural selection has ever done anything more spectacular than cause bacteria to develop drug-resistant strains, where is the overwhelming evidence that justifies assigning to it an ability we do not attribute to any other natural force in the universe: the ability to create order out of disorder?
Three types of evidence are cited: first, the fact that species are so well suited to their environments is offered as evidence that they have "adapted" to them. Of course, if they were not well-adapted, they would be extinct, and that would be offered as even stronger evidence against design. Second, they point to changes due to artificial selection, where intelligent humans select features already present in the gene pool, as evidence of what can be accomplished when natural forces select among genetic accidents. But, as always, the main evidence offered is the "evolutionary tree" of similarities connecting all species, fossil and living. These similarities were of course noticed long before Darwin (many animals have four legs, one head, two eyes and a tail!); all modern science has done is to show that the similarities go much deeper than those noticed by ancient man.
Although these similarities may, to our modern minds, suggest natural causes, they do not really tell us anything about what those causes might be. In fact, the fossil record does not even support the idea that new organs and new systems of organs arose gradually: new orders, classes and phyla consistently appear suddenly. For example, Harvard paleontologist George Gaylord Simpson in "The History of Life" [Simpson 1960] writes:
It is a feature of the known fossil record that most taxa appear abruptly. They are not, as a rule, led up to by a sequence of almost imperceptibly changing forerunners such as Darwin believed should be usual in evolution...This phenomenon becomes more universal and more intense as the hierarchy of categories is ascended. Gaps among known species are sporadic and often small. Gaps among known orders, classes and phyla are systematic and almost always large. These peculiarities of the record pose one of the most important theoretical problems in the whole history of life: Is the sudden appearance of higher categories a phenomenon of evolution or of the record only, due to sampling bias and other inadequacies?
An analogy may be useful here. If some future paleontologist were to unearth two species of Fords, he might find it plausible that one evolved gradually from the other through natural causes. He might find the lack of gradual transitions between automobile families more problematic, for example, in the transition from mechanical to hydraulic brake systems, or from manual to automatic transmissions, or from steam engines to internal combustion engines. He would be even more puzzled by the huge differences between the bicycle and motor vehicle phyla, or between the boat and airplane phyla. But if he is a Darwinist, heaven help us when he discovers motorcycles and Hovercraft, that will constitute spectacular confirmation of his theory that all forms of transportation arose gradually from a common ancestor, without design.
Since I am well aware that logic and evidence are powerless against the popular perception, nurtured by prestigious journals such as National Geographic and Nature, that no serious scientists harbor any doubts about Darwinism, I want to offer here a portion of a November 5, 1980 New York Times News Service report:
Biology's understanding of how evolution works, which has long postulated a gradual process of Darwinian natural selection acting on genetic mutations, is undergoing its broadest and deepest revolution in nearly 50 years. At the heart of the revolution is something that might seem a paradox. Recent discoveries have only strengthened Darwin's epochal conclusion that all forms of life evolved from a common ancestor. Genetic analysis, for example, has shown that every organism is governed by the same genetic code controlling the same biochemical processes. At the same time, however, many studies suggest that the origin of species was not the way Darwin suggested...Exactly how evolution happened is now a matter of great controversy among biologists. Although the debate has been under way for several years, it reached a crescendo last month, as some 150 scientists specializing in evolutionary studies met for four days in Chicago's Field Museum of Natural History to thrash out a variety of new hypotheses that are challenging older ideas. The meeting, which was closed to all but a few observers, included nearly all the leading evolutionists in paleontology, population genetics, taxonomy and related fields. No clear resolution of the controversies was in sight. This fact has often been exploited by religious fundamentalists who misunderstood it to suggest weakness in the fact of evolution rather than the perceived mechanism. Actually, it reflects significant progress toward a much deeper understanding of the history of life on Earth. At issue during the Chicago meeting was macroevolution, a term that is itself a matter of debate but which generally refers to the evolution of major differences, such as those separating species or larger classifications...Darwin suggested that such major products of evolution were the results of very long periods of gradual natural selection, the mechanism that is widely accepted today as accounting for minor adaptations...Darwin knew he was on shaky ground in extending natural selection to account for differences between major groups of organisms. The fossil record of his day showed no gradual transitions between such groups, but he suggested that further fossil discoveries would fill the missing links. 'The pattern that we were told to find for the last 120 years does not exist,' declared Niles Eldridge, a paleontologist from the American Museum of Natural History in New York. Eldridge reminded the meeting of what many fossil hunters have recognized as they trace the history of a species through successive layers of ancient sediments. Species simply appear at a given point in geologic time, persist largely unchanged for a few million years and then disappear. There are very few examples--some say none--of one species shading gradually into another."
Axioms and Evidence To fully appreciate why such an easily discredited theory is still so widely accepted today, we need to go back to an old 1888 book Evolution [Le Conte 1888] by Joseph Le Conte, professor of Geology and Natural History at the University of California,who writes:
"Intermediate links may be wanting now, but they must, of course, have existed once--i.e., in previous geological times, and therefore ought to be found fossil. In distribution in space or geographically, organic kinds may be marked off by hard-and-fast lines but, if their derivative origin be true, in their distribution in time or geologically, there ought to be many examples of insensible shadings between them. In fact, if we only had all the extinct forms, the organic kingdom, taken as a whole and throughout all time, ought to consist not of species at all, but simply of individual forms, shading insensibly into each other...But this is not the fact. On the contrary, the law of distribution in time is apparently similar in this respect to the law of distribution in space, already given. As in the case of contiguous geographical faunas, the change is apparently by substitution of one species for another, and not by transmutation of one species into another. So also in successive geological faunas, the change seems rather by substitution than by transmutation. In both cases species seem to come in suddenly, with all their specific characters perfect, remain substantially unchanged as long as they last, and then die out and are replaced by others. Certainly this looks much like immutability of specific forms, and supernaturalism of specific origin...The reason for this, given by Darwin and other evolutionists, is the extremely fragmentary character of the geological record...While it is true that there are many and wide gaps in the record...yet there are some cases where the record is not only continuous for hundreds of feet in thickness, but the abundance of life was very great, and the conditions necessary for preservation exceptionally good...and yet, although the species change greatly, and perhaps many times, in passing from the lowest to the highest strata, we do not usually, it must be acknowledged, find the gradual transitions we would naturally expect if the changes were effected by gradual transformations."
Le Conte also acknowledges that natural selection cannot explain the appearance of new features:
"...neither can it [natural selection] explain the first steps of advance toward usefulness. An organ must be already useful before natural selection can take hold of it to improve on it."
After acknowledging that the only direct evidence, the fossil record, does not support the idea of gradual change, and that the only theory ever taken seriously as to the causes of these changes cannot explain anything new, Le Conte nevertheless concludes:
"We are confident that evolution is absolutely certain--not evolution as a special theory--Lamarckian, Darwinian, Spencerian...but evolution as a law of derivation of forms from previous forms. In this sense it is not only certain, it is axiomatic ...The origins of new phenomena are often obscure, even inexplicable, but we never think to doubt that they have a natural cause; for so to doubt is to doubt the validity of reason, and the rational constitution of Nature."
Conclusions Science has been so successful in explaining natural phenomena that the modern scientist is convinced that it can explain everything, and anything that doesn't fit into this model is simply ignored. It doesn't matter that there were no natural causes before Nature came into existence, so he cannot hope to ever explain the sudden creation of time, space, matter and energy and our universe in the Big Bang. It doesn't matter that quantum mechanics is based on a "principle of indeterminacy", that tells us that every "natural" phenomenon has a component that is forever beyond the ability of science to explain or predict, he still insists nothing is beyond the reach of his science. When he discovers that all of the basic constants of physics, such as the speed of light, the charge and mass of the electron, Planck's constant, etc., had to have almost exactly the values that they do have in order for any conceivable form of life to survive in our universe, he proposes the "anthropic principle" [eg, Leggett 1987] and says that there must be many other universes with the same laws, but random values for the basic constants, and one was bound to get the values right. When you ask him how a mechanical process such as natural selection could cause human consciousness to arise out of inanimate matter, he says, "human consciousness -- what's that?" And he talks about human evolution as if he were an outside observer, and never seems to wonder how he got inside one of the animals he is studying. And when you ask how the four fundamental forces of Nature could rearrange the basic particles of Nature into libraries full of encyclopedias, science texts and novels, and computers, connected to laser printers, CRTs and keyboards and the Internet, he says, well, order can increase in an open system.
The development of life may have only violated one law of science, but that was the one Sir Arthur Eddington [Eddington 1929] called the "supreme" law of Nature, and it has violated that in a most spectacular way. At least that is my opinion, but perhaps I am wrong. Perhaps it only seems extremely improbable, but really isn't, that, under the right conditions, the influx of stellar energy into a planet could cause atoms to rearrange themselves into nuclear power plants and spaceships and computers. But one would think that at least this would be considered an open question, and those who argue that it really is extremely improbable, and thus contrary to the basic principle underlying the second law, would be given a measure of respect, and taken seriously by their colleagues, but we aren't.
References
* Angrist, S. and L.Hepler (1967) Order and Chaos, Basic Books.
* Behe, Michael (1996) Darwin's Black Box, Free Press.
* Daubenmire, R.F. (1947) Plants and Environment, John Wiley & Sons.
* Eddington, Arthur (1929) The Nature of the Physical World, McMillan.
* Ford, Kenneth (1968) Basic Physics, Blaisdell Publishing Co.
* Le Conte, Joseph (1888) Evolution, D.Appleton and Company.
* Leggett, A.J. (1987) The Problems of Physics, Oxford University Press.
* Rostand, Jean (1956) A Biologist's View, Wm. Heinemann Ltd.
* Sewell, Granville (2000) "A Mathematician's View of Evolution," The Mathematical Intelligencer 22, number 4, 5-7.
* Sewell, Granville (2001) "Can ANYTHING Happen in an Open System?," The Mathematical Intelligencer 23, number 4, 8-10.
* Sewell, Granville (2005) The Numerical Solution of Ordinary and Partial Differential Equations, second edition, John Wiley & Sons.
* Simpson, George Gaylord (1960) "The History of Life," in Volume I of Evolution after Darwin, University of Chicago Press.
TOPICS: Culture/Society; Editorial; Miscellaneous; Philosophy
KEYWORDS: crevolist; darwin; darwinsblackbox; evolution; id; irreduciblycomplex; secondlaw; thermodynamics
Navigation: use the links below to view more comments.
first previous 1-20, 21-40, 41-60, 61-63 next last
To: SirLinksalot
To: SirLinksalot
"In Appendix D of my new book [Sewell, 2005], I take a closer look at the equations for entropy change, which apply not only to thermal entropy but also to the entropy associated with anything else that diffuses, and show that they do not simply say that order cannot increase in a closed system, they also say that in an open system, order cannot increase faster than it is imported through the boundary. According to these equations, the thermal order in an open system can decrease in two different ways -- it can be converted to disorder, or it can be exported through the boundary. It can increase in only one way: by importation through the boundary. Similarly, the increase in "carbon order" in an open system cannot be greater than the carbon order imported through the boundary, and the increase in "chromium order" cannot be greater than the chromium order imported through the boundary, and so on."
Does anyone have the book?
From table of contents on amazon, one can see appendix D here:
http://www.amazon.com/gp/reader/0471735809/ref=sib_dp_top_toc/102-8612528-6088129?ie=UTF8&p=S007#reader-link
To: FreedomProtector
To: FreedomProtector; grey_whiskers
Based on that link, Sewell seems unaware that entropy is a state function. Randomness has little to do with the entropy; no given configuration is random, only processes are random.
44
posted on
10/23/2006 3:27:34 PM PDT
by
Doctor Stochastic
(Vegetabilisch = chaotisch ist der Charakter der Modernen. - Friedrich Schlegel)
To: Doctor Stochastic; grey_whiskers
Based on that link, Sewell seems unaware that entropy is a state function. Randomness has little to do with the entropy; no given configuration is random, only processes are random.
The thermodynamic state of a system is its condition as described by its physical characteristics. Temperature and internal energy are both state functions, the entropy function is a state function as well. Although normally expressed on a macroscopic scale (Clausius/Kelvin-Planck etc), it is also used in statistical mechanics, the microstate of that system is given by the exact position and velocity of every air molecule in the room.
Entropy can be defined as the logarithm of the number of microstates. The microstate and macroscopic scale can be shown to be related by "rigorously by using integrals over appropriately defined quantities instead of simply counting states".
Entropy is a description of the state of the position and velocity of the matter in the system. Configurations can be random or orderly.
From Physics for Scientists and Engineers, Third Edition, Raymond A Serway James 1992 Updated Printing
(undergraduate college level Physics book)
As you look around beauties of nature, it is easy to recognize that the events of natural processes have in them a large element of chance. For example, the spacing between treees in a natural forest is quite random. On the other hand, if you were to discover a forest where all the trees were equally spaced, you would probably conclude that the forest was man-made. Likewise, leaves fall to the ground with random arrangements. It would be highly unlikely to find the leaves laid out in perfectly straight rows or in one neat pile. We can express the results of such observations by saying that a disorderly arrangement is much more probable then an orderly one if the laws of nature are allowed to act without interference.
One of the main results of statistical mechanics is that isolated systems end toward disorder and entropy is a measure of disorder. In light of this new view of entropy, Boltzmann found that an alternative method for calculating entropy, S, is through use the the important relation
S = k ln W
where k is Boltzmann's constant, and W (not to be confused with work) is a number of proportional to the probability of the occurrence of a particular event..." ....several probability examples follow...... Consider a container of gas consisting of 10^23 molecules. If all of them were found moving in the same direction with the same speed at the some instant, the outcome would be similar to drawing marbles from the bag [half are red and are replaced] 10^23 times and finding a a red marble on every draw. This is clearly an unlikely set of events.
Temperature and internal energy are both state functions, the entropy function is a state function as well. Entropy is a description of the probability of a given configuration or a "logarithm of the number of microstates".
One of the main results of statistical mechanics is that isolated systems end toward disorder and entropy is a measure of disorder.
There are many more interesting properties of entropy the second law gives us one defines isentropic, adiabatic, irreversibility etc...
Sewell adds: "According to these equations, the thermal order in an open system can decrease in two different ways -- it can be converted to disorder, or it can be exported through the boundary. It can increase in only one way: by importation through the boundary."
Sewell's tautology that "if an increase in order is extremely improbable when a system is closed, it is still extremely improbable when a system is open, unless something is entering which makes it not extremely improbable" is a simply a more general statement.
Although, the application of vector calculus is obvious as boundaries are defined for thermodynamic systems, Sewell's mathematical deduction and resulting tautology is brilliant and gives us another interesting practical property of entropy deduced from the second law in addition to the ones related to isentropic, adiabatic, irreversibility etc which we already know.
Thanks for the ping, Doctor Stochastic!
To: FreedomProtector
it is also used in statistical mechanics, the microstate of that system is given by the exact position and velocity of every air molecule in the room. ???
Are we assuming no internal degrees of freedom (monatomic gases)?
Do you happen to remember the term "partition function"?
Cheers!
To: grey_whiskers
Sloppy sentence. My bad.
The intent was not to provide a comprehensive overview of statistical mechanics, merely an introduction to the relation of entropy to statistical mechanics.
Are we assuming no internal degrees of freedom (monatomic gases)? The author is merely assuming "air" and adds that it is a 'simple' example. While some elements of air are monatomic (ex noble gases), the other elements (obviously more) are diatomic elements hydrogen, nitrogen, oxygen etc...
"Entropy is a relationship between macroscopic and microscopic quantities. To illustrate what I mean by that statement I'm going to consider a simple example, namely a sealed room full of air. A full, microscopic description of the state of the room at any given time would include the position and velocity of every air molecule in it."
Wikipedia provides the example of a classical ideal diatomic gas.....
Do you happen to remember the term "partition function"? The partition function is still a statement of probability that a system occupies or does not occupy a particular microstate
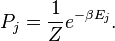
Thermodynamics can be used to describe the probability of a certain arrangements of entities compared to another, and which general direction those arrangments are most likely to move in without interference. While the definition of orderly is vague, it is clear there are orderly arrangments which we observe which it is absurd to assume that a natural process organized them. "a forest where all of the trees were equally spaced, you would probably conclude that the forest was man-made" or "leaves laid out in perfectly straight rows or in one neat pile. We can express the results of such observations by saying that a disorderly arrangement is much more probable then an orderly one if the laws of nature are allowed to act without interference." from the
3rd edition of this book (The edition I own)
There is an Organizer outside of nature who interfered when the world was created.
To: grey_whiskers
A mathematical deduction beginning with the statistical mechanics definition of entropy to demonstrate the same statement as Sewell would be interesting.
"According to these equations, the thermal order in an open system can decrease in two different ways -- it can be converted to disorder, or it can be exported through the boundary. It can increase in only one way: by importation through the boundary."
quod erat demonstrandum would be satisfying statement at the end, I suspect the deduction would be fairly rigorous.
To: FreedomProtector
should be "some other elements" not "other elements"
To: Oberon
2. It's outside the realm of science to consider the existence of some intelligence that manipulates the mutation of organisms. As long as you're busy building false assumptions, here's another one. Science does this sort of stuff all the time, including with guided mutation of organisms. So it's clearly not outside the realm of science to do this stuff.
One wonders why science is therefore claimed to be utterly incapable of detecting what science can do. Dogmatic convenience, perhaps?
50
posted on
10/24/2006 8:24:55 AM PDT
by
r9etb
To: Doctor Stochastic
Randomness has little to do with the entropy; no given configuration is random, only processes are random. With a name like Dr. Stochastic, you come up with this? C'mon, doc.... Suppose there's a configuration C(t), subjected to a random process. Are you really gonna make the claim that you know C(t+1) with probability=1? Because that's what your comment seems to be saying.
51
posted on
10/24/2006 8:30:40 AM PDT
by
r9etb
To: r9etb
One wonders why science is therefore claimed to be utterly incapable of detecting what science can do. Dogmatic convenience, perhaps? No, that wassn't my point at all. I didn't mean to address the possibility or lack thereof of manipulating genes; I meant to point out that the minute you postulate an intelligence manipulating speciation in the wild, you've gone out of science and into philosophy.
Nothing I said was intended as an ideological attack. Please don't take it that way.
52
posted on
10/24/2006 8:35:09 AM PDT
by
Oberon
(What does it take to make government shrink?)
To: FreedomProtector
To: r9etb
No. That's not what I said. I said that the configuration C(t+1) has the same entropy whether or not it is obtained from C(t) by random or non-random processes.
54
posted on
10/24/2006 9:01:53 AM PDT
by
Doctor Stochastic
(Vegetabilisch = chaotisch ist der Charakter der Modernen. - Friedrich Schlegel)
To: Oberon
I meant to point out that the minute you postulate an intelligence manipulating speciation in the wild, you've gone out of science and into philosophy. Nothing I said was intended as an ideological attack. Please don't take it that way. I didn't take it that way, actually. The question itself is directed toward the oft-cited claim that it's "philosophy" rather than science -- esentially a claim that identifying design is scientifically impossible. Aside from the obvious -- we do it all the time -- the claim itself appears to be unscientific, and based on the assumption that we can't detect it. It raises the question of whether or not it's cited as a matter of dogmatic convenience.
The problem with the claim is that it ignores the fact that doing the manipulation sits firmly in the realm of science. It makes very little sense to claim that science can do something, but is then incapable of then detecting what it did. It might well be difficult to do so, especially long after the fact. But an alleged inability to detect the results of scientific manipulation would be due to a weakness in the detecting science. And an inability to detect long-ago design is scientifically explainable as well.
If we do suppose that the detection of design really does belong in the philosophy dept., would it not then be logically necessary to relegate the identification of "non-design" to the same dept.?
55
posted on
10/24/2006 9:04:16 AM PDT
by
r9etb
To: r9etb
If we do suppose that the detection of design really does belong in the philosophy dept., would it not then be logically necessary to relegate the identification of "non-design" to the same dept.? Absolutely! In fact, I've made that very point on an ID thread just a few weeks ago. Naturalism is itself a faith.
56
posted on
10/24/2006 9:12:24 AM PDT
by
Oberon
(What does it take to make government shrink?)
To: Oberon
Absolutely! In fact, I've made that very point on an ID thread just a few weeks ago. Naturalism is itself a faith. I'd go about halfway with that ... absolute naturalism is a matter of faith, just as absolute creationism is.
But here in the real world, we've got a more complex reality where we know that some things occur naturally; and we know that it's possible for things to be designed for specific purposes -- including, increasingly, biological entities.
I recall once reading something by Einstein, about his thought processes in coming up with Special Relativity. His first step was to investigate the assumptions under which science was currently operating.
It seems to me that the assumption of "random processes only" is open to investigation -- certainly evolutionary biology treats it as a given. But it is up to those who do not consider it a "given" to establish sufficient grounds to revise the assumption.
57
posted on
10/24/2006 9:52:06 AM PDT
by
r9etb
To: Doctor Stochastic
No. That's not what I said. I said that the configuration C(t+1) has the same entropy whether or not it is obtained from C(t) by random or non-random processes.
If is it a closed system and a random process, it is unlikely to have the same entropy, it will likely have more entropy, and it will never ever have less.
Sewell adds that if it is an open system "According to these equations, the thermal order in an open system can decrease in two different ways -- it can be converted to disorder, or it can be exported through the boundary. It can increase in only one way: by importation through the boundary."
To: FreedomProtector
If is it a closed system and a random process, it is unlikely to have the same entropy, it will likely have more entropy, and it will never ever have less. No. Entropy is a state function. Random, non-random, deterministic, chaotic, quantum, spooky, etc., all processes lead to the same entropy for the same state. This is what Sewell seems to be missing in his appendix.
59
posted on
10/24/2006 3:15:14 PM PDT
by
Doctor Stochastic
(Vegetabilisch = chaotisch ist der Charakter der Modernen. - Friedrich Schlegel)
To: Doctor Stochastic
If is it a closed system and a random process, it is unlikely to have the same entropy, it will likely have more entropy, and it will never ever have less.
No. Entropy is a state function. Random, non-random, deterministic, chaotic, quantum, spooky, etc., all processes lead to the same entropy for the same state. This is what Sewell seems to be missing in his appendix.
I stated the second law of thermodynamics and you said "No"?
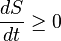
Of course entropy is a state function. It is a measure of the thermodynamic state at a particular point in time. If time x occurs before time y, the state at time x will always be have less or equal entropy to the state of entropy at time y, for a closed system.
"all processes lead to the same entropy for the same state"
The state changes with time. That is like saying the entropy at time x is the same as the entropy time x. Maybe all of your systems are assumed to already be at equlibrium, but even then the statement is a strech. Maybe you have invented a way to stop time (travel the speed of light), but I doubt it.
Navigation: use the links below to view more comments.
first previous 1-20, 21-40, 41-60, 61-63 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson