Posted on 05/26/2021 7:21:31 AM PDT by Red Badger

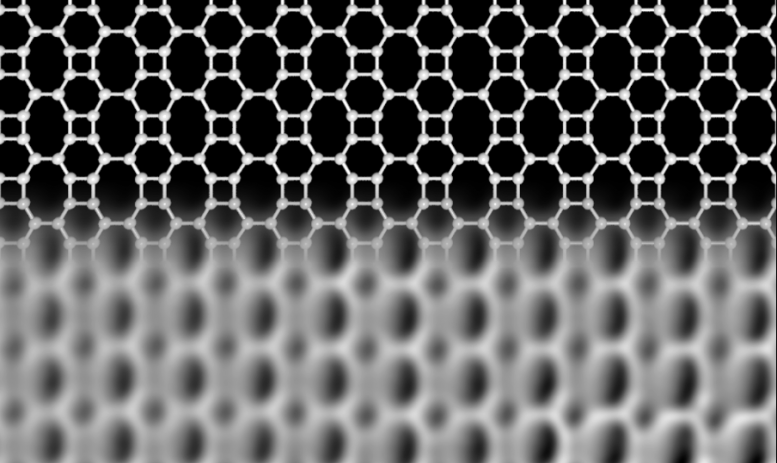
Structure of the new carbon network. The upper part shows schematically the linking of the carbon atoms, forming squares, hexagons, and octagons. The lower part is an image of the network, obtained with high-resolution microscopy. Credit: University of Marburg, Aalto University
================================================================================
Carbon exists in various forms. In addition to diamond and graphite, there are recently discovered forms with astonishing properties. For example graphene, with a thickness of just one atomic layer, is the thinnest known material, and its unusual properties make it an extremely exciting candidate for applications like future electronics and high-tech engineering. In graphene, each carbon atom is linked to three neighbors, forming hexagons arranged in a honeycomb network. Theoretical studies have shown that carbon atoms can also arrange in other flat network patterns, while still binding to three neighbors, but none of these predicted networks had been realized until now.
Researchers at the University of Marburg in Germany and Aalto University in Finland have now discovered a new carbon network, which is atomically thin like graphene, but is made up of squares, hexagons, and octagons forming an ordered lattice. They confirmed the unique structure of the network using high-resolution scanning probe microscopy and interestingly found that its electronic properties are very different from those of graphene.
In contrast to graphene and other forms of carbon, the new Biphenylene network — as the new material is named — has metallic properties. Narrow stripes of the network, only 21 atoms wide, already behave like a metal, while graphene is a semiconductor at this size. “These stripes could be used as conducting wires in future carbon-based electronic devices.” said professor Michael Gottfried, at University of Marburg, who leads the team who developed the idea. The lead author of the study, Qitang Fan from Marburg continues, “This novel carbon network may also serve as a superior anode material in lithium-ion batteries, with a larger lithium storage capacity compared to that of the current graphene-based materials.”
The team at Aalto University helped image the material and decipher its properties. The group of Professor Peter Liljeroth carried out the high-resolution microscopy that showed the structure of the material, while researchers led by Professor Adam Foster used computer simulations and analysis to understand the exciting electrical properties of the material.
The new material is made by assembling carbon-containing molecules on an extremely smooth gold surface. These molecules first form chains, which consist of linked hexagons, and a subsequent reaction connects these chains together to form the squares and octagons. An important feature of the chains is that they are chiral, which means that they exist in two mirroring types, like left and right hands. Only chains of the same type aggregate on the gold surface, forming well-ordered assemblies, before they connect. This is critical for the formation of the new carbon material, because the reaction between two different types of chains leads only to graphene. “The new idea is to use molecular precursors that are tweaked to yield biphenylene instead of graphene” explains Linghao Yan, who carried out the high-resolution microscopy experiments at Aalto University.
For now, the teams work to produce larger sheets of the material, so that its application potential can be further explored. However, “We are confident that this new synthesis method will lead to the discovery of other novel carbon networks.” said Professor Liljeroth.
Reference: “Biphenylene network: A nonbenzenoid carbon allotrope” by Qitang Fan, Linghao Yan, Matthias W. Tripp, Ondrej Krejcí, Stavrina Dimosthenous, Stefan R. Kachel, Mengyi Chen, Adam S. Foster, Ulrich Koert, Peter Liljeroth and J. Michael Gottfried, 21 May 2021, Science. DOI: 10.1126/science.abg4509
Carbon is nature’s Legos.
That may prove to be true in the uncomplimentary sense that graphene and similar materials never amount to more than laboratory toys with little practical value.
And Mr. Qitang Fan is funneling all this information back to Xi Jinping and the CCP. It seems that anywhere serious cutting edge research is being done, there is at least one Chinese agent in attendance. And that’s why they will continue to eat OUR lunch.
Tech moves to allow us to manipulate these new discoveries. As it does, those toys become something very useful. Don’t discount the way these things all of a sudden are everywhere.
Self landing rockets were joked about, Kevlar and Teflon were nothing until they became regular everyday items. Computing power and human genome, DNA testing, advanced tech is out there.
Ban carbon!
I get the onward and upward rationale, but a lot of what appears in the news media as scientific advances is hyped and slow to deliver if they deliver at all. No personal robots, flying cars, or fusion power yet despite decades of supposed advances.
I get the onward and upward through rationale, but a lot of what appears in the news media as scientific advances is hyped and slow to deliver if they deliver at all. No personal robots, flying cars, or fusion power yet despite decades of supposed advances. New materials make new products and devices possible, but the path is neither quick nor certain.
“ That may prove to be true in the uncomplimentary sense that graphene and similar materials never amount to more than laboratory toys with little practical value.
I have a couple of these great graphene batteries that work just as advertised.
5 amp hour, 60 watts, that fully recharges from empty in 17 - 18 minutes.
Real Graphene Power Bank 5000 mAh 60W 【17 Minutes Full Charge】 Super Fast Charging, Portable, Lightweight, Graphene Battery Pack for iPhone, Galaxy Note10+, Nintendo Switch, iPad Pro and More
by Real Graphene USA
Learn more: https://www.amazon.com/dp/B08DC5WZR5/ref=cm_sw_em_r_mt_dp_2PGG4NQ9TH5G5H5R14H4?_encoding=UTF8&psc=1
-Easy
An acquaintance at church may differ. He has over 100 patents, some covering graphene applications.
That is very cool. I’ll probably look into it next time I need a power bank. I have several right now. One is 30k mAh. Once that one finally gives up the ghost, it will be time for another.
Sweet. What is the price point though? My guess is that it is too high to displace lithium batteries in electric vehicles.
Permit me to offer a few words of appreciation for tech entrepreneurs like Elon Musk and the legal and business infrastructure and skilled workers who convert scientific advances into valuable products and services. Without them, most of those advances would be little more than geek chatter.
I suspect that if you wanted to build a tesla battery out of these at their retail price point, it would cost you more than the car.
That said, as these are among the first commercially available Graphene Batteries, they should currently enjoy a large profit margin.
Nothing in them makes them inherently much more expensive than common LiFePo batteries. It turns out that Graphene is not all that hard to make.
And they DO charge FAST, compared to normal lithium batteries.
~Easy
Apparently, most graphene batteries still use lithium, with the graphene incorporated into an improved cathode.
That is correct. All of them, so far, are just better lithium batteries due to improved conductivity provided by the Graphene coating on the cathode.
Patents are not the same as products, in fact there are at least two patents on magnetic motors according the Popular mechanics some 40 years ago.
Excellent metaphor
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.