Posted on 07/27/2020 7:48:04 AM PDT by SeekAndFind
According to new research published in the preprint server medRxiv* in July 2020, the use of hydroxychloroquine (HCQ) among outpatients in clinical trials, without high-risk factors for cardiac arrhythmia, is safe, with gastrointestinal side-effects being the most common side effects and no fatal adverse outcomes.

As the COVID-19 pandemic continues all around the world, with over 630,000 deaths so far, scientific research has focused on finding effective medications against the virus. A strong contender, right from the very beginning, has been HCQ, mainly because of the heavy backing given by political heavyweights.
HCQ has been demonstrated to have in vitro antiviral activity against SARS-CoV-2, the virus that causes COVID-19, and hinders its replication. However, correspondingly strong evidence of its activity in the treatment or prevention of this disease has not been found so far.
For this reason, the World Health Organization and several other health organizations are conducting studies on the safety and efficacy of this drug in both asymptomatic or mildly symptomatic cases, as well as sicker patients in hospitals.
Earlier research has reported an increase in the reported incidence of cardiac side effects with the use of HCQ and azithromycin in combination. This led to the revocation of the Emergency Authorization issued by the US Food and Drug Authority (FDA) for the use of HCQ in severely ill COVID-19 patients.
Instead, it said, “Hydroxychloroquine and chloroquine can cause abnormal heart rhythms such as QT interval prolongation and…ventricular tachycardia,” especially among those using HCQ with azithromycin or those who already had kidney or heart problems.
The issue with QT prolongation is the risk of ventricular arrhythmias. Most of these episodes in the context of HCQ use have been when the drug was used with another arrhythmogenic drug, used for an extended period or at excessive doses.
However, HCQ has been in clinical use, and when used as per the guidelines, for people without such issues, it is a useful drug in multiple disciplines and has been used in autoimmune rheumatic conditions. Baseline laboratory tests or ECG monitoring are rarely carried out in most cases.
However, health organizations have drawn attention to its ability to cause sudden deaths, especially when coupled with azithromycin. In most cases, safety issues have arisen in sicker, hospitalized patients who have severe disease, or other coexisting disease conditions, where multiple medications are taken at the same time.
There are reasons to suspect that this may not reflect the normal course of events following the use of HCQ. For instance, the current virus may gain entry into cardiac cells and cause damage, and even arrhythmias, as reported in many cases.
Another potential cause of arrhythmias may be the elevated cytokines, especially in patients who already have heart damage. HCQ in current trials is also used at much higher doses than for the conditions it is currently approved for.
Finally, there may be significant disruption of normal electrolyte levels, which, in combination with kidney failure, makes the individual more prone to arrhythmias. This means that separate safety tests need to be carried out specifically for COVID-19 patients. HCQ is thought to be safer in outpatients with COVID-19 compared with hospitalized patients.
The current study focuses on the safety evaluation of HCQ in outpatients, compiling data from three randomized controlled trials, where HCQ was used as pre-exposure prophylaxis, post-exposure prophylaxis, and early treatment, respectively. The researchers excluded all participants with contraindications to the use of this drug.
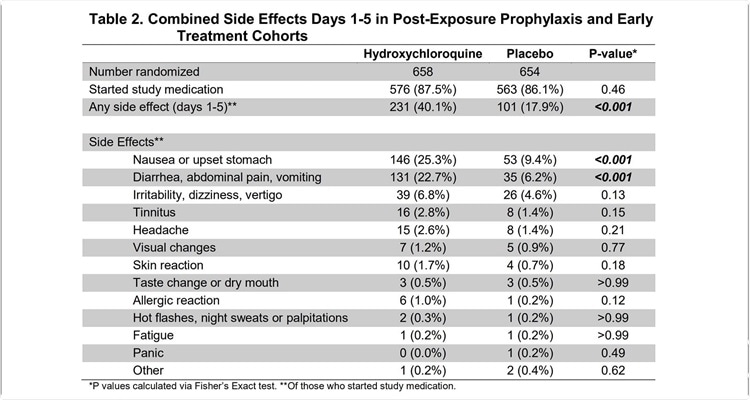
The study included approximately 2,800 individuals, with a median age of 40 years. About 60% of them were in good health. The researchers found that side effects were reported in around 85% of the participants, and 27% had one or more side effects related to the medication.
When the dosage frequency was compared, they found that about 30%, 35%, 30%, and 20% had side effects when the drug was administered daily, twice a week, once a week or when a placebo was used.
Around 30% of patients reported side effects in the trials where the drug was administered post-exposure or post-infection.
The most common side effects reported were gastrointestinal, namely, stomach upset or nausea, diarrhea, vomiting, or abdominal pain. Overall, these symptoms were reported in a quarter of patients on daily dosage (about 20% and 15% with twice-weekly or weekly dosage), and in 10% of those on placebo.
However, in the post-exposure placebo vs. treatment groups, the rate of gastrointestinal side effects was comparable. The side effects were rated as tolerable and not requiring the cessation of medication.
Other reported side effects included lightheadedness or dizziness and allergic reactions.
There were only two instances of cardiac arrhythmias in the whole patient population, one of which occurred in the placebo group, the other in a patient taking HCQ twice a week. There were no reported deaths in this low-risk population.
The current study excluded hospitalized, presumably sicker, patients, who were mostly older. Most of the participants were health workers, and reasonably well-informed about health. Therefore, the safety of HCQ concerning its use in severely ill COVID-19 patients remains unclear.
Ongoing clinical trials can safely continue with research participants and regulatory bodies reassured as to the general safety of hydroxychloroquine when using appropriate exclusion criteria.”
Ping as per your request
Oh hell nahhhh....that can’t be correct....ask dr falsie and all the talking head drs in the msm...
18 percent side effects from sugar pill say a lot about the people involved, hypochondria anyone?
Thanks for posting this!
As I’ve stated in the past, HCQ is very safe. When I was in Korea in the seventies, there was a bottle of HCQ on every table in the mess hall with instructions on how many pills to take and when. The HCQ was right next to the salt and pepper shakers. None of us were under a doctor’s supervision. None of us were in the hospital. To the best of my knowledge no one keeled over after taking HCQ.
In other words, they'll figure it out sometime after the election, maybe
A friend of mine who runs a ministry in Tanzania told me that there have been few cases of covid there because everyone in the country takes chloroquine. Take that MSM doubters.
A friend of mine who runs a ministry in Tanzania told me that there have been few cases of covid there because everyone in the country takes chloroquine. Take that MSM doubters.
But Dr Joy Behar said it was deadly poison, akin to swallowing cyanide.
Ping as per your request
Thank you!!!!!
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.