Posted on 04/16/2020 8:03:10 PM PDT by SeekAndFind
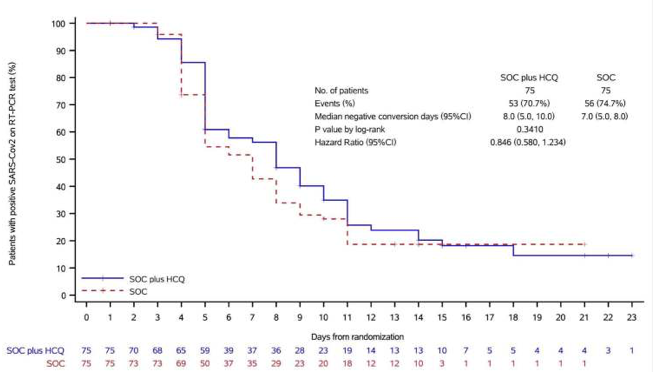
A just-reported Chinese study compares the clinical outcomes of COVID-19 patients treated with hydroxychloroquine with those of patients receiving standard of care. The results, alas, are disappointing.
I summarized that study in my roundup yesterday of COVID-19 therapeutic research, pointing out that this randomized controlled trial of 150 patients "found no difference in the rate of viral load reduction or symptom alleviation between the group treated with hydroxychloroquine and the one that had not been." Now the University of Vermont pulmonologist Josh Farkas has published his own analysis of the results, delving more deeply into the data.
The patients in both arms of the study were well-matched demographically and clinically, Farkas notes. Most suffered relatively mild cases of the disease, and treatment was initiated fairly late—about 16 to 17 days after disease onset. Twenty-eight days into the trial, the researchers found essentially no difference between the two cohorts with respect to the percent of patients in which the virus was undetectable.

Farkas adds:
This endpoint most directly addresses the question: does hydroxychloroquine exert anti-viral activity in vivo? The answer seems to be: nope. Even if the drug were administered too late to affect the clinical course of the infection, if it exerted any anti-viral activity then we might expect to see that effect here. If anything, there might be a trend towards delayed viral clearance in patients treated with hydroxychloroquine.
The study also found that fever and respiratory symptoms did not abate any faster in patients who had been treated with hydroxychloroquine.
Farkas acknowledges the study's limits, including its small size and that the researchers were not blinded—that is, they knew which patients were being given the treatment. "Nonetheless," he says, "this study currently represents the highest available quality of evidence regarding hydroxychloroquine."
"For now, the best available evidence does not support the use of hydroxychloroquine in COVID-19," Farkas concludes. "It seems prudent to restrict the use of hydroxychloroquine to randomized controlled studies for the time being." He does acknowledge that future studies in which COVID-19 patients are treated earlier in the course of their infections may yet find that hydroxychloroquine offers some therapeutic benefits. Fingers crossed.
Chinese Study....Bwahahahahahahahaha!
Ok China.
Chinese study three days to travel the world will not infect the world with Coronavirus.
Chinese study??????????????????????????????????
Did they include zinc, or did they just use HCQ?
Trusting anything that comes out of China shows lack of REASON. (pun intended)
” ..treatment was initiated fairly late..”
Vs. instructions for Hydroxy.., use early.
Hey I gotta Chinese bridge for sale cheap. $6 bucks both ways. Start the bidding.
ChiCom study to throw off the world with misinformation. Again.
Get the HCQ with Zpac and zinc to victims early and they don’t get hospitalized and they don’t die.
I trust Dr. Zelenko’s results among hundreds from Monroe, NY even without a study than the ChiCom release.
We have too many stories of individuals doing well after the HCQ cocktail.
nonsense study- period- many many trials and studies have been done now- almost lal of them showing very very promising results- only these ‘chinese studies’ show anything ‘negative’
According to this analysis by Josh Farkas associate professor of Pulmonary and Critical Care Medicine at the University of Vermont, on the Chinese study,
https://emcrit.org/pulmcrit/hydroxychloroquine-fail/
It says:
The study has major limitations, for example:
* Lack of blinding or placebo control.
* Performance of the study by a contract research organization, rather than directly by the investigators.
* Early termination due to recruitment problems and the impression of benefit (which may have led to under-powering).
* Relatively long delay between symptom onset and treatment initiation (16-17 days).
The last limitation is the one that kills the result of the study.
According to this analysis by Josh Farkas associate professor of Pulmonary and Critical Care Medicine at the University of Vermont, on the Chinese study,
https://emcrit.org/pulmcrit/hydroxychloroquine-fail/
It says:
The study has major limitations, for example:
* Lack of blinding or placebo control.
* Performance of the study by a contract research organization, rather than directly by the investigators.
* Early termination due to recruitment problems and the impression of benefit (which may have led to under-powering).
* Relatively long delay between symptom onset and treatment initiation (16-17 days).
The last limitation is the one that kills the result of the study.
I’ll pass. Chinese would steal it from me anyway. 8>)
150 Patients!!!!
Hardly worth doing study.
Ever.
![]()
![]()
![]()
![]()
A Chinese study?
The author is a jack@$$
thanks for that info- yep- that last one is a doosey-
many docs though are touting it’s usage even in late stages, and a lot of anecdotal stories about people on brink making miraculous recoveries when given the drugs- but I’m sure some don’t make it in late stages-
[[”Nonetheless,” he says, “this study currently represents the highest available quality of evidence regarding hydroxychloroquine.”]]
BS- man6 studies and trials show just the opposite- and were more thorough than their study- evidently-
The new "OK Boomer"...
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.