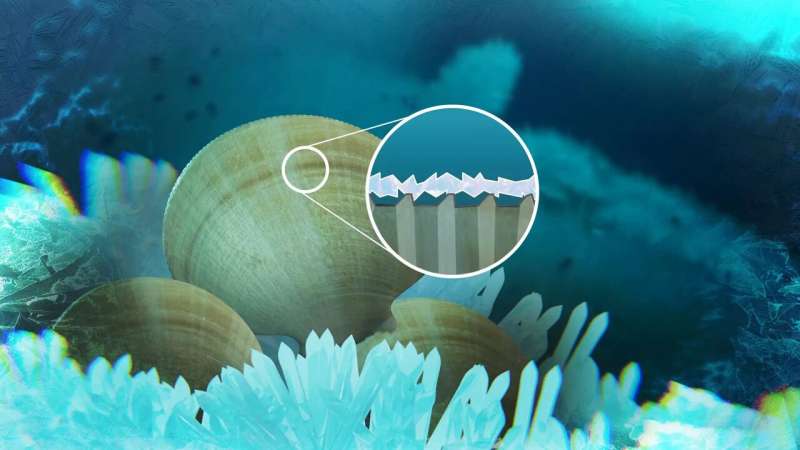
Due to a regular surface structure on the mussel "Adamussium colbecki" ice adheres to it only very weakly and can be easily washed away by currents. Credit: Max Planck Institute for Polymer Research
Posted on 03/03/2022 11:42:54 AM PST by LibWhacker

Due to a regular surface structure on the mussel "Adamussium colbecki" ice adheres to it only very weakly and can be easily washed away by currents. Credit: Max Planck Institute for Polymer Research
Antarctic waters have conditions in which objects and living creatures can freeze even under water. This is a major problem for marine travel in polar regions. So-called supercooled water has a temperature just below the freezing point. Due to the high salt content, water in Antarctica has a freezing point of about -1.9 °C, but is about 0.05 °C colder. The smallest disturbances such as grains of sand or surfaces can cause this supercooled water to freeze—with sometimes fatal consequences for creatures that cannot survive frozen.
The Antarctic scallop "Adamussium colbecki" resists this, as chemist Konrad Meister knows. Meister is a professor at the University of Alaska and heads a research group in Mischa Bonn's department at the Max Planck Institute for Polymer Research (MPI-P) in Mainz. During an expedition in Antarctica, divers drew his attention to the scallop with the efficient ice protection mechanism. "Our divers reported that they had never observed large-scale ice on the surface of this native scallop species," Meister says.
The international research team, consisting of members of several MPI-P research groups as well as the University of Oregon, suspects that the scallop species developed a special surface structure during evolution that protects it from icing. While scallops in warmer regions have disordered or smooth shell surfaces, the Antarctic species has a microscopic, very regular structure.
The microscope reveals small ridges that run in a radiating pattern on their shell. These ridges ensure that water freezes preferentially there. If the freezing process continues, a continuous layer of ice forms, resting only on the ridges. Due to the low adhesion between ice and shell, the smallest underwater flow can therefore wash off the ice again and the scallop does not freeze.
In addition to microscope studies, the research team also conducted icing experiments with the Antarctic and with a scallop from warmer regions. It was found that far less force is needed to remove the ice layer on the Antarctic scallop than for the other species.
"It is exciting how evolution has obviously given this scallop an advantage," says Konrad Meister. "New technological applications based on the principle of bionics are conceivable from the knowledge of the ice-free shell. For example, non-icing surfaces could be highly interesting for polar shipping."
I notice they mentioned application of this to polar shipping, but NOT to reducing icing on aircraft wings, e.g., which seems to me, a non-expert on all things, to be a far more important technological application. Hmm... perplexing. But interesting anyway.
Can you think of other possibly important applications for this "discovery"?
This could be used to prevent icing on power lines so they do not get weighed down.
THAT’S a good one, thanks!
Brought to you and me, and all of us, by the lowly mussel.
There have been several attempts at making ice-free surfaces for various applications such as aviation. Many of them involve using engineered nanostructured surfaces.
The problem with nanostructured surfaces, or even larger, microscopic surface features is that they wear off very quickly. Kind of like Rain-X on you windshield. It lasts for a while but gradually small bits of dust blast the coating away.
I have a project at work which involves freezing a solution. We wanted to use metal molds but have found ice sticks to all metals and even Teflon. Can’t use nanostructured surfaces because the molds would wear out too quickly.
Seriously, thank you.

The article doesn’t make any sense. Anything will freeze whenever it’s temperature goes below it’s own freezing point. It doesn’t matter what the condition of its surrounding material is. It could be solid, liquid, or gas. The article seems to indicate that the mussels’ surface can shed ice particles easily, but it doesn’t explain at why it doesn’t itself freeze.
Puget Sound Mussels simmered in coconut milk and lemon grass.
Mighty fine eatin’.
I love scallops.
Could you use a thin metal mold, then briefly heat it enough that the ice comes loose enough to be taken out?
My understanding... It freezes, but it doesn’t adhere. It flakes off easily.
Yes that can be done, as it is for popsicles. However we have found a blast freezer can freeze so quickly that silicone molds work fine. The trick is the air must flow quickly to remove heat rapidly.
Yum, I want some! I LOVE seafood. Wife hates it. She accommodates me. I accommodate her. So we never eat it, LOL.
These mussels are stressed and not thinking Warm
I’m hungry.
Puget Sound Mussels simmered in coconut milk and lemon grass.
Mighty fine eatin’.
—
Puget Sound Mussels, having filtered through the secondary sewage treatments release into the Sound. Chop away.
There is so much sewage in the Sound that underwater looks like floating bits of TP, but are really algae blooms on even smaller bits of s...t paper. Salmon are forced into the upper water columns and become easy prey for Seals and Sealions
That sounds like a lot of bull scat.
Suspect the sound is a lot cleaner than it was 40-50 years ago when sewer pipes went straight out.
That sounds like a lot of bull scat.
—
Nope - Sea/Tac only does secondary treatment - you must have tertiary to produce clean water (a very expensive process - one which few cities do). Secondary releases lots of particulate matter, which produces blooms in warm weather. Just ask any one who uses nets to fish about algae blooms and the origins.
Nonsense.

Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.