Posted on 09/09/2018 7:19:16 AM PDT by ETL
Scientists have reached a “milestone” in a technique of semi-artificial photosynthesis that could eventually create an “unlimited source of renewable energy,” according to a new study.
Artificial photosynthesis has been around for decades, but scientists haven’t been able to develop it on a scale large enough to support an industrial level, or that could operate without the use of expensive or polluting devices.
Semi-artificial photosynthesis, a relatively new field of study, aims to address those concerns by combining manmade technologies with biological processes in order to mimic nature’s method of splitting water into oxygen and hydrogen.
In the latest study, researchers at the University of Cambridge focused on an enzyme found in algae called Hydrogenase – which has lied dormant for millennia. Their findings were published Sept. 3 in Nature Energy.
“Hydrogenase is an enzyme present in algae that is capable of reducing protons into hydrogen,” Katarzyna Sokól, first author of the study, said in a statement.
“During evolution, this process has been deactivated because it wasn’t necessary for survival but we successfully managed to bypass the inactivity to achieve the reaction we wanted — splitting water into hydrogen and oxygen.”
The hydrogenase also dramatically improves the amount of energy that’s produced and stored. Sokól believes this new process will enable new innovations in the world of renewable energy.
In addition to developing new technologies, these types of studies are essential for the future of space travel — as scientists continue to figure out the most efficient ways to keep spacecrafts running on deep space voyages.
(Excerpt) Read more at foxnews.com ...

2 H2O —> 2 H2 + O2
Efficient and economical photochemical water splitting would be a technological breakthrough that could underpin a hydrogen economy. A version of water splitting occurs in photosynthesis, but hydrogen is not produced. No practical version of water splitting has been demonstrated, but the two component reactions (H2 production and O2 production) are well known. The reverse of water splitting is the basis of the hydrogen fuel cell.
Electrolysis:
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen (H2) due to an electric current being passed through the water.[1]
Atmospheric electricity utilization for the chemical reaction in which water is separated into oxygen and hydrogen. (Image via: Vion, US patent 28793. June 1860.)
Vion, U.S. Patent 28,793, “Improved method of using atmospheric electricity”, June 1860.
In power to gas production schemes, the excess power or off peak power created by wind generators or solar arrays is used for load balancing of the energy grid by storing and later injecting the hydrogen into the natural gas grid.
Electrolysis of water ship Hydrogen Challenger
Production of hydrogen from water is energy intensive. Potential electrical energy supplies include hydropower, wind turbines, or photovoltaic cells. Usually, the electricity consumed is more valuable than the hydrogen produced so this method has not been widely used.
In contrast with low-temperature electrolysis, high-temperature electrolysis (HTE) of water converts more of the initial heat energy into chemical energy (hydrogen), potentially doubling efficiency to about 50%. Because some of the energy in HTE is supplied in the form of heat, less of the energy must be converted twice (from heat to electricity, and then to chemical form), and so the process is more efficient.
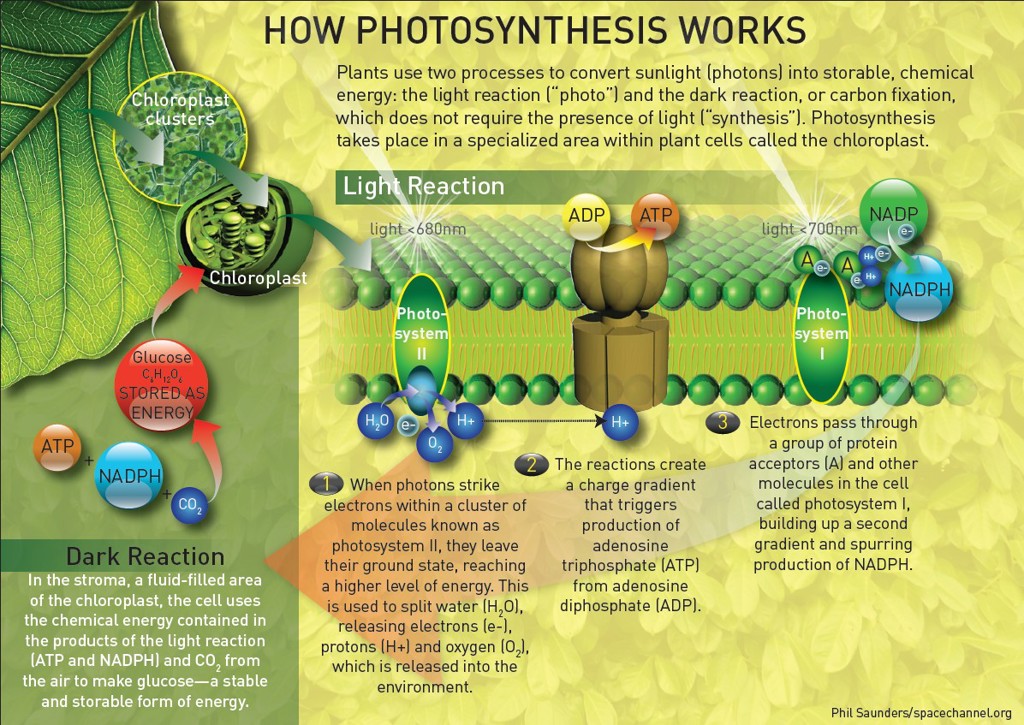
Water splitting in photosynthesis:
A version of water splitting occurs in photosynthesis, but the electrons are shunted, not to protons, but to the electron transport chain in photosystem II. The electrons are used to convert carbon dioxide into sugars.
When photosystem I gets photo-excited, electron transfer reactions gets initiated, which results in reduction of a series of electron acceptors, eventually reducing NADP+ to NADPH and PS I is oxidized. The oxidized photosystem I captures electrons from photosystem II through a series of steps involving agents like plastoquinone, cytochromes and plastocyanine.
The photosystem II then brings about water oxidation resulting in evolution of oxygen, the reaction being catalyzed by CaMn4O5 clusters embedded in complex protein environment; the complex is known as oxygen evolving complex (OEC).[2][3]
In biological hydrogen production, the electrons produced by the photosystem are shunted not to a chemical synthesis apparatus but to hydrogenases, resulting in formation of H2. This biohydrogen is produced in an bioreactor.[4]
Photoelectrochemical water splitting
(main articles: photoelectrochemical cell and artificial photosynthesis)
Using electricity produced by photovoltaic systems potentially offers the cleanest way to produce hydrogen, other than nuclear, wind, geothermal, and hydroelectric. Again, water is broken down into hydrogen and oxygen by electrolysis, but the electrical energy is obtained by a photoelectrochemical cell (PEC) process. The system is also named artificial photosynthesis.[5][6][7][8]
Photocatalytic water splitting:
(main article: Photocatalytic water splitting)
The conversion of solar energy to hydrogen by means of water splitting process is one of the most interesting ways to achieve clean and renewable energy.
However, if this process is assisted by photocatalysts suspended directly in water rather than a photovoltaic or an electrolytic system, the reaction takes place in one step, it therefore can be more efficient.[9][10]
Radiolysis:
(main article: Radiolysis § Hydrogen_production)
Nuclear radiation routinely breaks water bonds, in the Mponeng gold mine, South Africa, researchers found in a naturally high radiation zone, a community dominated by a new phylotype of Desulfotomaculum, feeding on primarily radiolytically produced H2.[11] Spent nuclear fuel/”nuclear waste” is also being looked at as a potential source of hydrogen.
Thermal decomposition of water:
(main article: Thermochemical cycle)
In thermolysis, water molecules split into their atomic components hydrogen and oxygen. For example, at 2200 °C about three percent of all H2O are dissociated into various combinations of hydrogen and oxygen atoms, mostly H, H2, O, O2, and OH. Other reaction products like H2O2 or HO2 remain minor.
At the very high temperature of 3000 °C more than half of the water molecules are decomposed, but at ambient temperatures only one molecule in 100 trillion dissociates by the effect of heat.[12] The high temperatures and material constraints have limited the applications of this approach.
Nuclear-thermal:
(see also: NGNP)
Some prototype Generation IV reactors, such as the High-temperature engineering test reactor, operate at 850 to 1000 degrees Celsius, considerably hotter than existing commercial nuclear power plants.
General Atomics predicts that hydrogen produced in a High Temperature Gas Cooled Reactor (HTGR) would cost $1.53/kg. In 2003, steam reforming of natural gas yielded hydrogen at $1.40/kg. At 2005 gas prices, hydrogen cost $2.70/kg.[citation needed] Hence, just within the United States, a savings of tens of billions of dollars per year is possible with a nuclear-powered supply. Much of this savings would translate into reduced oil and natural gas imports.
One side benefit of a nuclear reactor that produces both electricity and hydrogen is that it can shift production between the two. For instance, the plant might produce electricity during the day and hydrogen at night, matching its electrical generation profile to the daily variation in demand.
If the hydrogen can be produced economically, this scheme would compete favorably with existing grid energy storage schemes. What is more, there is sufficient hydrogen demand in the United States that all daily peak generation could be handled by such plants.[13]
The hybrid thermoelectric Copper-chlorine cycle is a cogeneration system using the waste heat from nuclear reactors, specifically the CANDU supercritical water reactor.[14]
Solar-thermal:
The high temperatures necessary to split water can be achieved through the use of concentrating solar power. Hydrosol-2 is a 100-kilowatt pilot plant at the Plataforma Solar de Almería in Spain which uses sunlight to obtain the required 800 to 1,200 °C to split water.
Hydrosol II has been in operation since 2008. The design of this 100-kilowatt pilot plant is based on a modular concept. As a result, it may be possible that this technology could be readily scaled up to megawatt range by multiplying the available reactor units and by connecting the plant to heliostat fields (fields of sun-tracking mirrors) of a suitable size.[15]
Material constraints due to the required high temperatures are reduced by the design of a membrane reactor with simultaneous extraction of hydrogen and oxygen that exploits a defined thermal gradient and the fast diffusion of hydrogen.
With concentrated sunlight as heat source and only water in the reaction chamber, the produced gases are very clean with the only possible contaminant being water. A “Solar Water Cracker” with a concentrator of about 100 m² can produce almost one kilogram of hydrogen per sunshine hour.[16]
All this science I don’t understand,
It’s just my job five days a week...
—Elton John
as scientists continue to figure out the most efficient ways to keep spacecrafts running on deep space voyages.
—
It is called a nuclear fission reactor.
I’m betting on Number 6 fuel oil.

The hydrogenase also dramatically improves the amount of energy that’s produced and stored. Sokól believes this new process will enable new innovations in the world of renewable energy.
...
Science by press release reliably amounts to nothing. It’s usually a researcher creating a lot of smoke and light in order to get more funding.
The problem is that the sun produces only so much energy per square unit of the earth’s surface. The only way to increase the energy per demand is to allocate more acreage to solar farms.
[....that could eventually ....]
Signed the Kanamits.
Howard Johnson: "You know, Nietzsche said, 'Out of chaos comes order.'"
Olson Johnson: "Oh, blow it out your a$$, Howard!"
I have learned out in the real world that you don’t ever get something for free. It is called the law of conservation of energy and by extension, matter.
To Serve Man
Series: The Twilight Zone
Writer: Rod Serling, based on the story by Damon Knight
Director: Richard L. Bare
Apparently benign alien emissaries show mankind how to end the misery of war, plague and famine. The Kanamits, nine-foot tall aliens, arrive on Earth with one lofty goal: To Serve Man. They end war, they end famine. They make the military wonder: what's the catch?
CAST: Richard Kiel, Hardie Albright, Robert Tafur, Lloyd Bochner, Lomax Study, Theodore Marcuse, Susan Cummings, Nelson Olmstead.
Watch it, or any other TZ episode, online for free at:
http://www.cbs.com/classics/the_twilight_zone/
I’m betting on Number 6 fuel oil.
—
Not for traveling thru space or supplying much of anything as oil is far too heavy to pack around as an energy source off earth.
Or fusion.
Thanks ETL.



Yes and no.
H2 can be stored in various forms. As the solar energy is cumulative, it becomes possible to store vast amounts of energy.
H2 gas has a tendency though to embrittle its containment. There are ways to store H2 though that do not embrittle its containment.
H2 containers become the focus and the cost therein.
If true ..... Arkancide.

Making fuel on site for a return trip to Mars may be a step closer. A cunning way to split water into oxygen and hydrogen in two distinct steps could be a boon to both astronauts and future Earthlings, enabling them to use renewable energy sources for making hydrogen fuel.
Hydrogen fuel cells can power vehicles ranging from cars to submarines and rockets. They can also heat buildings, and double as portable power-packs for computers or other kit used in the field. But existing methods for creating usable hydrogen gas from water require a lot of electricity. That means renewable energy sources like wind or sunlight, which are often patchy, are not reliable enough.
It can also be hazardous to scale up “artificial leaves”, which make fuel from sunlight, just like plants, says Lee Cronin at the University of Glasgow, UK. This is because the low powers available don’t produce the gases quickly enough to keep them apart once they form. “All they do is build up oxygen and hydrogen until they explode,” he says.
Cronin and his colleagues see this as a major obstacle to a future in which hydrogen fuel replaces oil. To get around it, they built a device that uses a single pulse of power to split water, so continuous energy is not needed.
Catch and release
The device zaps water with electricity to release oxygen, then a silicon-based chemical mediator dissolved in the water mops up stray protons and electrons. When it is full, the mediator turns blue, letting a human operator know it can be removed and stored for later.
When the hydrogen is needed, putting the mediator in contact with a platinum catalyst allows those electrons and protons to recombine to make hydrogen gas.
The whole process uses a single whack of power, and patchy renewable energy will suffice for this, says Cronin. In return, he says, 30 times as much hydrogen can be made than from existing systems. The device could find uses generating power in developing countries or for making fuel on Mars to power a rocket back to Earth.
It is unclear whether Cronin’s device will be able to compete with other existing processes, says Steve Reece, a water-splitting expert at Lockheed Martin in Cambridge, Massachusetts. “It will be interesting to see how this concept scales.”
https://www.newscientist.com/article/dn26204-water-splitter-could-make-hydrogen-fuel-on-mars/
Also it should be noted that processes that have evolved on earth should be considered inefficient. Pure science will offer the best solution.
For example: if we had modeled our aircraft around the flapping of bird wing, high speed would have never been achieved. The bird wing was optimized for a specialized task and is not optimized for our needs.
fusion reactors do not exist may as well just use zero-point energy.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.