Posted on 06/07/2009 3:37:45 PM PDT by neverdem
The discovery of a bimetallic palladium(III) complex that can catalyse formation of carbon-heteroatom bonds adds a new facet to our understanding of the chemistry of one of the most widely-used metals in catalysis, say US chemists.
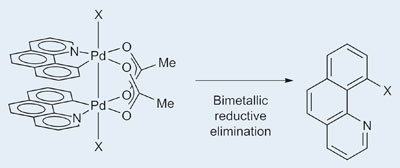
Tobias Ritter and David Powers from Harvard University, Massachusetts, have shown for the first time that a palladium(III) complex is responsible for conversion of a C-H bond to a C-Cl bond. While such reactions were known before, the nature of the catalyst had not been studied in detail. 'This work was about analysis of how this kind of transformation works,' says Ritter, 'and we found this Pd(III) complex, which is not what people would have expected. It's generally accepted that these reactions go through Pd(II)/Pd(IV) cycles.'
The team found that when bimetallic palladium(II) complexes, made from palladium acetate and benzoquinoline, were oxidised with halogens or hypervalent iodine reagents they formed Pd(III) complexes with a central metal-metal bond. Ritter explains that these complexes can undergo bimetallic reductive elimination to chlorinate one of the benzoquinoline molecules. In more usual monometallic systems, reductive elimination is a process whereby a bond is formed between two of the ligands on the metal - two electrons are shifted to the metal centre and its oxidation state is therefore reduced by two (for example Pd(II) becomes Pd(0)). In this case, each metal centre handles a single electron, with both being reduced from Pd(III) to Pd(II).

|
Each palladium atom is oxidised by one electron, which results in a Pd(III)-Pd(III) single bond. During bimetallic reductive elimination, an overall two-electron process, each palladium atom is reduced by one electron, which results in concomitant cleavage of the Pd-Pd bond.
© Nature Chemistry
|
Ritter believes that cooperation between the two metal centres makes this reaction pathway more favourable: 'We're distributing the electronic workload between two metals and getting energy out of the Pd-Pd bond,' he explains, 'which might be the reason why it goes through Pd(III) and not Pd(IV).'
To prove that the bimetallic complexes really were responsible for the catalysis, Ritter performed kinetic experiments using complexes where the two carboxylate groups, which bridge between the metal centres, were either on separate acetate molecules or tethered together as arms of a single ligand. If the complex was disproportionating into a combination of monometallic Pd(IV) and Pd(II) species, different kinetic profiles would be expected under these two sets of conditions. 'We don't see this,' says Ritter, 'so the two palladium atoms must stay together.'
Robin Bedford, an expert in catalysis from the University of Bristol, UK, is very impressed by the paper: 'It's a really elegant result, with very thorough and convincing mechanistic studies,' he says. The crucial factor, Bedford adds, is that the results fit very well with observations of catalytic reactions, and that the reaction mixtures are typical of those currently being used in catalysis, which adds evidence to the possibility that these palladium(III) complexes are truly involved in the catalytic activity.
This is just the very first step,' says Ritter, 'at this point we do not know how general this bimetallic palladium(III) scaffold will be. We are interested in using the mechanistic insight we have gained to develop new reactions - we can think about things in a way now that we could not before.'
Bedford agrees that the work opens up a lot of possibilities: 'I think this will have a significant impact, from a mechanistic perspective, on the larger field of C-H activation under oxidative conditions,' he says, 'and of course, mechanism and extension of that mechanism into new reactions go hand-in-hand, so I think this could open up some really exciting new catalytic chemistry too.'
Phillip Broadwith
Interesting? Spread the word using the 'tools' menu on the left.
D C Powers and T Ritter, Nature Chemistry, 2009. DOI: 10.1038/NCHEM.246
I have not studied it in detail, but at this point I can say “terrific. We’ve found a new way to make dioxin, and other chlorinated hydrocarbons.”
Cheers!
Despite eco-nutcase propaganda, the mere inclusion of a C-Cl bond doesn't automatically generate a toxic material. The ability to put such bonds in precise locations will boost the ability to more efficiently make things like drugs.
Good find. Thanks for the ping.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.