Posted on 04/25/2022 10:03:50 AM PDT by Red Badger

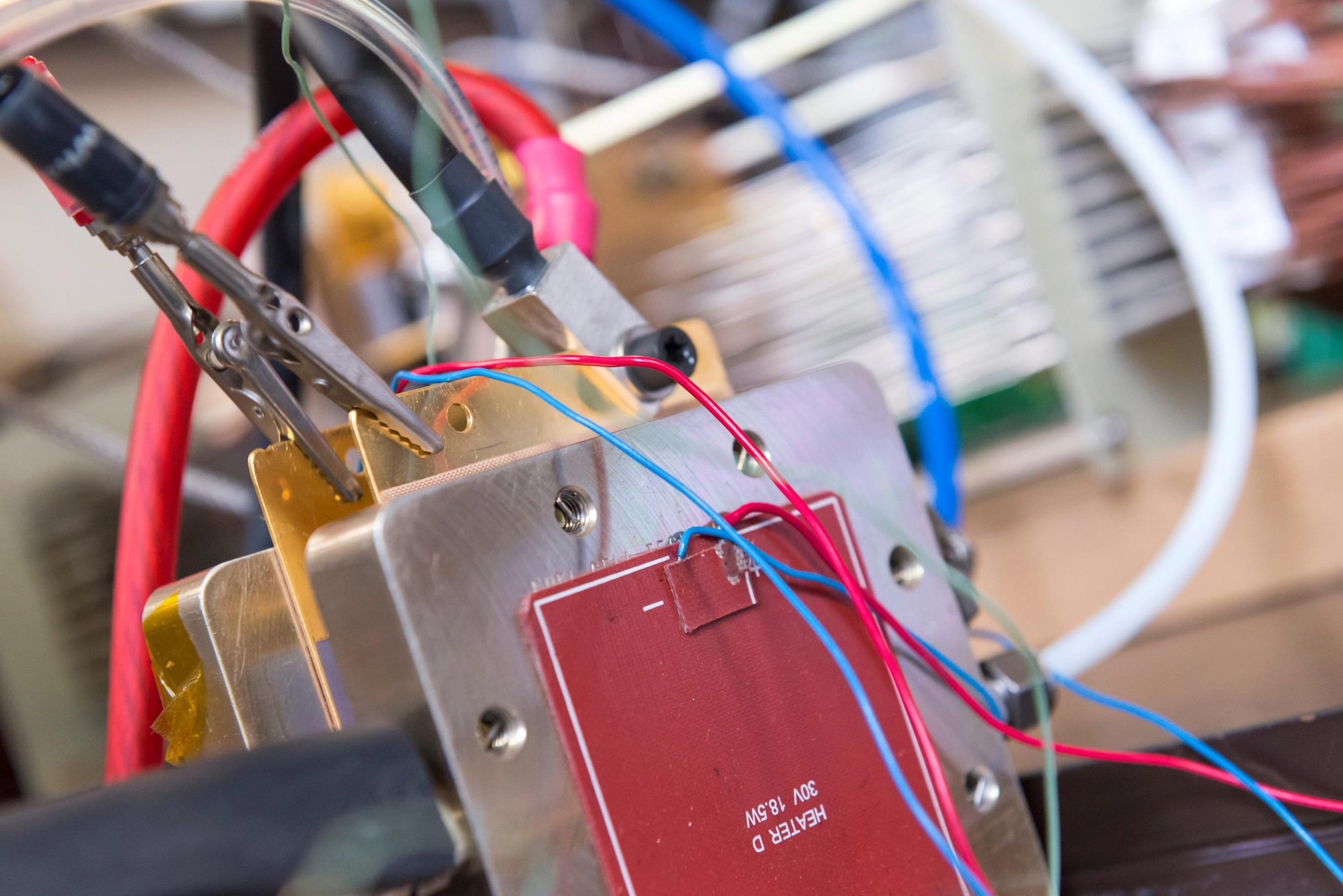
Fuel Cell Being Tested The new fuel cell being tested in the lab. Credit: Imperial College London
Imperial researchers have developed a new hydrogen fuel cell that uses iron instead of rare and costly platinum, enabling greater use of the technology.
Hydrogen fuel cells convert hydrogen to electricity with just water vapor as a byproduct, making them an appealing green alternative for portable power, particularly for vehicles.
However, the expense of one of the primary components has impeded its broad adoption. The fuel cells rely on a catalyst made of platinum, which is expensive and scarce, to assist the reaction that generates power.
Now, a European team led by Imperial College London researchers has created a catalyst using only iron, carbon, and nitrogen – materials that are cheap and readily available – and shown that it can be used to operate a fuel cell at high power. Their results are published today (April 25, 2022) in Nature Catalysis.
Lead researcher Professor Anthony Kucernak, from the Department of Chemistry at Imperial, said: “Currently, around 60% of the cost of a single fuel cell is the platinum for the catalyst. To make fuel cells a real viable alternative to fossil-fuel-powered vehicles, for example, we need to bring that cost down.
“Our cheaper catalyst design should make this a reality, and allow deployment of significantly more renewable energy systems that use hydrogen as fuel, ultimately reducing greenhouse gas emissions and putting the world on a path to net-zero emissions.”
The team’s innovation was to produce a catalyst where all the iron was dispersed as single atoms within an electrically conducting carbon matrix. Single-atom iron has different chemical properties than bulk iron, where all the atoms are clustered together, making it more reactive.
These properties mean the iron boosts the reactions needed in the fuel cell, acting as a good substitute for platinum. In lab tests, the team showed that a single-atom iron catalyst has performance approaching that of platinum-based catalysts in a real fuel cell system.
As well as producing a cheaper catalyst for fuel cells, the method the team developed to create could be adapted for other catalysts for other processes, such as chemical reactions using atmospheric oxygen as a reactant instead of expensive chemical oxidants, and in the treatment of wastewater using air to remove harmful contaminants.
First author Dr. Asad Mehmood, from the Department of Chemistry at Imperial, said: “We have developed a new approach to make a range of ‘single atom’ catalysts that offer an opportunity to allow a range of new chemical and electrochemical processes. Specifically, we used a unique synthetic method, called transmetallation, to avoid forming iron clusters during synthesis. This process should be beneficial to other scientists looking to prepare a similar type of catalyst.”
The team collaborated with UK fuel cell catalyst manufacturer Johnson Matthey to test the catalyst in appropriate systems and hope to scale up their new catalyst so it can be used in commercial fuel cells. In the meantime, they are working to improve the stability of the catalyst, so it matches platinum in durability as well as performance.
Reference: “High loading of single atomic iron sites in Fe–NC oxygen reduction catalysts for proton exchange membrane fuel cells” 25 April 2022, Nature Catalysis.
DOI: 10.1038/s41929-022-00772-9
I was pointing out that there should be some platinum available for the new fuel cells on cars with the deletion of the catalytic converter from cars. Since the article doesn’t go into detail about how much is required to create the fuel cells, and I have no knowledge as to how much is used in a catalytic converter this should provide some cost benefit.
Do they both require the same amount of platinum, or more, or less? The cost savings will vary on those questions.
I used to work with some Real Smart People who were members of the Chicago Catalysis Club. It sounds funny til you understand how important catalysts are to just about everything. Don’t like being told to buy an EV? Better love your catalysts then, cuz those big honkin things you see driving past a refinery aren’t called “cat crackers” because they meow.
And so far we're talking about industrial scale stuff. That is the problem. Fossil fuels work very well at industrial scale levels too until the gubment makes it hard to use them. Edison cells or any other kind of future power will suffer the same fate.
The only power applications I'm interested in are the ones I can do myself.
In both instances the quantity is small, but at today’s price of 915+ dollars an ounce it don’t take much..............
Way, way, way down just around the corner .... see that speck in the distance?
Interesting. But the drawback to “green hydrogen” is that it is too costly and too hard to manage. Never be scalable for any intended use, other than niches.
Water vapor is by far the biggest 'greenhouse' gas in our atmosphere.
And the most powerful....................
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.