Posted on 02/09/2022 12:47:12 PM PST by Red Badger

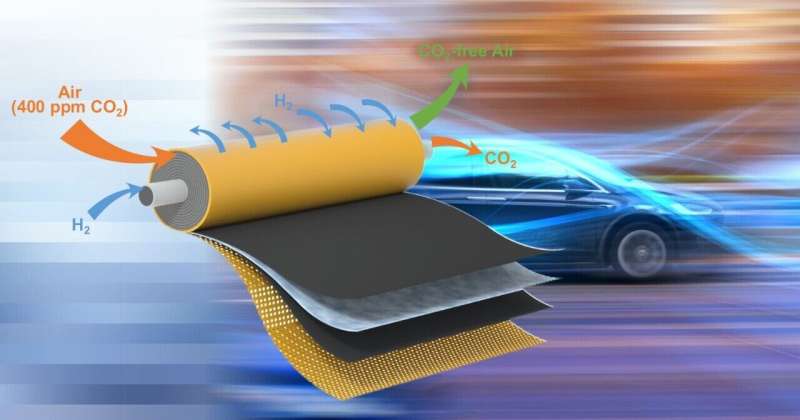
University of Delaware researchers have broken new ground that could bring more environmentally friendly fuel cells closer to commercialization. Credit: Graphic illustration by Jeffrey C. Chase
=========================================================================
University of Delaware (UD) engineers have demonstrated a way to effectively capture 99% of carbon dioxide from air using a novel electrochemical system powered by hydrogen.
It is a significant advance for carbon dioxide capture and could bring more environmentally friendly fuel cells closer to market.
The research team, led by UD Professor Yushan Yan, reported their method in Nature Energy on Thursday, February 3.
Game-changing tech for fuel cell efficiency
Fuel cells work by converting fuel chemical energy directly into electricity. They can be used in transportation for things like hybrid or zero-emission vehicles.
Yan, Henry Belin du Pont Chair of Chemical and Biomolecular Engineering at UD, has been working for some time to improve hydroxide exchange membrane (HEM) fuel cells, an economical and environmentally friendly alternative to traditional acid-based fuel cells used today.
But HEM fuel cells have a shortcoming that has kept them off the road—they are extremely sensitive to carbon dioxide in the air. Essentially, the carbon dioxide makes it hard for a HEM fuel cell to breathe.
This defect quickly reduces the fuel cell's performance and efficiency by up to 20%, rendering the fuel cell no better than a gasoline engine. Yan's research group has been searching for a workaround for this carbon dioxide conundrum for over 15 years.
A few years back, the researchers realized this disadvantage might actually be a solution—for carbon dioxide removal.
"Once we dug into the mechanism, we realized the fuel cells were capturing just about every bit of carbon dioxide that came into them, and they were really good at separating it to the other side," said Brian Setzler, assistant professor for research in chemical and biomolecular engineering and paper co-author.
While this isn't good for the fuel cell, the team knew if they could leverage this built-in "self-purging" process in a separate device upstream from the fuel cell stack, they could turn it into a carbon dioxide separator.
"It turns out our approach is very effective. We can capture 99% of the carbon dioxide out of the air in one pass if we have the right design and right configuration," said Yan.
So, how did they do it?
They found a way to embed the power source for the electrochemical technology inside the separation membrane. The approach involved internally short-circuiting the device.
"It's risky, but we managed to control this short-circuited fuel cell by hydrogen. And by using this internal electrically shorted membrane, we were able to get rid of the bulky components, such as bipolar plates, current collectors or any electrical wires typically found in a fuel cell stack," said Lin Shi, a doctoral candidate in the Yan group and the paper's lead author.
Now, the research team had an electrochemical device that looked like a normal filtration membrane made for separating out gases, but with the capability to continuously pick up minute amounts of carbon dioxide from the air like a more complicated electrochemical system.
In effect, embedding the device's wires inside the membrane created a short-cut that made it easier for the carbon dioxide particles to travel from one side to the other. It also enabled the team to construct a compact, spiral module with a large surface area in a small volume. In other words, they now have a smaller package capable of filtering greater quantities of air at a time, making it both effective and cost-effective for fuel cell applications. Meanwhile, fewer components mean less cost, and more importantly, provide a way to easily scale up for the market.
The research team's results showed that an electrochemical cell measuring 2 inches by 2 inches could continuously remove about 99% of the carbon dioxide found in air flowing at a rate of approximately two liters per minute. An early prototype spiral device about the size of a 12-ounce soda can is capable of filtering 10 liters of air per minute and scrubbing out 98% of the carbon dioxide, the researchers said.
Scaled for an automotive application, the device would be roughly the size of a gallon of milk, Setzler said, but the device could be used to remove carbon dioxide elsewhere, too. For example, the UD-patented technology could enable lighter, more efficient carbon dioxide removal devices in spacecraft or submarines, where ongoing filtration is critical.
"We have some ideas for a long-term roadmap that can really help us get there," said Setzler.
According to Shi, since the electrochemical system is powered by hydrogen, as the hydrogen economy develops, this electrochemical device could also be used in airplanes and buildings where air recirculation is desired as an energy-saving measure. Later this month, following his dissertation defense, Shi will join Versogen, a UD spinoff company founded by Yan, to continue advancing research toward sustainable green hydrogen.
Co-authors on the paper from the Yan lab include Yun Zhao, co-first author and research associate, who performed experimental work essential for testing the device; Stephanie Matz, a doctoral student who contributed to the designing and fabrication of the spiral module, and Shimshon Gottesfeld, an adjunct professor of chemical and biomolecular engineering at UD. Gottesfeld was principal investigator on the 2019 project, funded by the Advanced Research Projects Agency-Energy (ARPA-E), that led to the findings.
Explore further
Stackable artificial leaf uses less power than lightbulb to capture 100 times more carbon than other systems More information: Lin Shi et al, A shorted membrane electrochemical cell powered by hydrogen to remove CO2 from the air feed of hydroxide exchange membrane fuel cells, Nature Energy (2022). DOI: 10.1038/s41560-021-00969-5 Journal information: Nature Energy Provided by University of Delaware
LOL! Need a bigger one than that!
A tree?
There's nothing wrong with the Global CO2 composition.
Why would I start doing that NOW?
“If only they had this on Apollo 13.”
A lot of thick heads on the thread.
The A13 astronauts were dying from overdosing on CO2.
This invention sounds like a perfect scrubbing technology.
Not going to be a problem. I understand people are saving up old tyres to burn at ceremony.
Because they want all plant life to die???
—
I’ve read during each of the Earth’s Ice Ages, CO2 drops down near extinction-event levels.
CO2 notwithstanding, H2 fuel cells are only a way to extract energy that’s been stored there by a man-made process. The hydrogen is just another form of battery that you have to charge from a primary source. Coal, natgas, nuclear. No, solar and wind ain’t EVER going to be enough.
I read the article and it seems clear they’re talking about a filter on the intake to a fuel cell to reduce the CO2 going in. It doesn’t remove CO2 from the atmosphere. It just removes it from the intake so the fuel cell works better.
That said, I can see a remake of Aliens where they explain that this is how the Yutani-Weyland atmosphere processors work to terraform a planet or an environmentally sensitive season 4 episode of Star Trek Picard where a planet is using them to reverse the effects of a “primitive capitalistic fossil-fuel burning society.
Mass extinction event.
Then everything dies.
I once asked my hubby (some years ago), what would happen if we got rid of all of the Carbon Dioxide. His response was "all the plants would die". I then asked what would happen then. He replied, "we die".
This is really the biggest hoax ever perpetrated on the planet. Carbon Dioxide is LIFE! Cannot one person with a science background say this publicly?
LOL What are the two biggest sources of commercially produced hydrogen? Natural gas and methane extracted in oil refineries. So the fuel cells will use hydrogen extracted in CO2 producing processes to capture CO2. LOL
May i add that if there is a shortage of CO2, there will be a shortage of plant life.
Better think this thing through.
5.56mm
Of course if we just remove half of all the CO2 in the atmosphere, we will have killed every living thing on the planet. Maybe that’s the goal.
[[The cuurent CO2 percentage in the atmodphere is 0.04%]]
Man’s CO2 amounts to just 0.00136% of the atmosphere- I also think that that 0.4% is all greenhouse gases in addition to CO2? but man’s contribution amounts to nothing really-
[[If the atmosphere were represented by 100,000 people in a stadium, CO2 would fill... 41 seats.]]
Seats used up by man’s CO2 would be ... 1.36
Another wasy to look at it- man’s CO2 would be like putting 4 5 gallon pails of 100 degree water into an olympic sized pool of 90 degree water, and saying “It’s causing catastrophic Warming”
It’s not of cours-e the 4 pails of water reach equilibrium very quickly- because there is nowhere near enough to overwhelm the total amount of pool water and warm it up- Same with the atmosphere- CO2 heat reaches equilibrium very quickly- because there is just nowhere near enough CO2 molecules to keep the heat emanating into the atmosphere
This sounds like a nifty addition to an on-demand type of hydrogen fuel cell for vehicles.
The idiots want to kill all plants on the planet Earth.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.