Skip to comments.
The Most Comprehensive Assault On 'Global Warming' Ever
dailywire.com ^
| December 23, 2015
| Mike Van Biezen
Posted on 12/26/2015 9:54:31 PM PST by Tennessean4Bush
It made sense. Knowing that CO2 is a greenhouse gas and that our industrialized world is adding a large amount of it to the atmosphere on a yearly basis, I accepted the premise that this would cause global temperatures to rise. But one day about 7 years ago, I looked at the ubiquitous graph showing the "global" temperature of the last 150 years and noticed something odd. It was subtle, and as I found out later, disguised so that it would be overlooked. There appeared to be a period of about 40 years between 1940 and 1980 where the global temperatures actually declined a bit. As a data analysis expert, I could not ignore that subtle hint and began to look into it a little more. Forty years is a long time, and while carbon dioxide concentrations were increasing exponentially over the same period, I could not overlook that this showed an unexpected shift in the correlation between global temperatures and CO2 concentrations. Thus I began to look into it a little further and here are some of the results 7 years later.
Before we begin, let's establish what we know to be correct. The global average temperature has increased since the 1980's. Since the 1980's glaciers around the world are receding and the ice cap of the Arctic Ocean has lost ice since the 1980's, especially during the summer months. The average global temperature for the last 10 years is approximately 0.35 degrees centigrade higher than it was during the 1980's. The global warming community has exploited these facts to "prove" that human activity (aka burning of fossil fuels) is the cause of these increasing temperatures...
(Excerpt) Read more at dailywire.com ...
TOPICS: Extended News; Front Page News; News/Current Events
KEYWORDS: climatechange; globalwarming; hoax
Navigation: use the links below to view more comments.
first previous 1-20 ... 41-60, 61-80, 81-100, 101-110 next last
To: a fool in paradise
81
posted on
12/28/2015 1:28:26 AM PST
by
Gene Eric
(Don't be a statist!)
To: Rightwing Conspiratr1
82
posted on
12/28/2015 5:36:23 AM PST
by
Tennessean4Bush
(An optimist believes we live in the best of all possible worlds. A pessimist fears this is true.)
To: Reno89519
I am surprised that drive-up windows at fast food joints haven’t been banned.
To: Bob434
the heat is rising fro mthe surface of the earth- most of it does not even contact CO2 Convection is different. It is one of the two types of heat transfer that bypass CO2 (the other is latent heat transfer). It doesn't matter what level of CO2 there is, convection and latent transfer are independent. But radiative transfer is photons emitted from the surface and lower levels of the atmosphere and all* those photons are intercepted by CO2 (* a trivial number make it through without being intercepted)
IF there were a thick blanket of CO2 preventing heat from going past the CO2
With PV=nRT and R of 0.287, T of 300K, V of 1 liter, and P of 1 atmosphere; there are 0.01 moles of air in that liter or 6 x 10^21 molecules. Since 0.04% of those molecules are CO2, there are 10^18 CO2 molecules of CO2 in just a liter of atmosphere. That's a lot of molecules and a lot of chances to intercept an IR photon. The fact that it is such a low percentage (0.04%) is mitigated by the fact that air has so many molecules.
only a few billion tons of CO2
Only a few billion? It's actually 720 billion tonnes of CO2 and even though it is spread out there are still 10^18 molecules in every liter or 10^21 in a cubic meter.
84
posted on
12/28/2015 4:58:51 PM PST
by
palmer
(Net "neutrality" = Obama turning the internet over to foreign enemies)
To: Bob434
Let’s assume that at 1:00 pm the atmosphere is say, for the sake of illustration only, 100 degrees. This local area of atmosphere has just 0.04% CO2 in it- It captures upward IR photons, releases them, the energy results in heat of say, for the sake of illustration, 102 degrees- This 0.04% molecules of warmer heat collides with the mass of 99.96% of the area’s 100 degree molecules- instantly the 0.04% warmer molecules get cooled down to the 100 degrees Decent description, but two problems with the scenario: 1) the CO2 molecules get way warmer than 102F and 2) the 0.04% also warm the other molecules by a bit as they are cooled (has to go both ways).
85
posted on
12/28/2015 5:01:36 PM PST
by
palmer
(Net "neutrality" = Obama turning the internet over to foreign enemies)
To: palmer
[[720 billion]]
Yep which is 0.04% of the total mass of atmosphere
[[But radiative transfer is photons emitted from the surface and lower levels of the atmosphere and all* those photons are intercepted by CO2]]
This is what I’m not understanding- the atmosphere has just 0.04% CO2 in it- all the IR photons can’t possibly be intercepted by CO2- There isn’t nearly enough CO to capture more than a scant amount- You claimed 10^41 CO2 molecules (there are 10^44 total molecules). That would indicate nearly the whole atmosphere being comprised of CO2- Science tells us just 0.04% is comprised of CO2- where are you coming up with more than 90% being CO2?
[[1) the CO2 molecules get way warmer than 102F and 2) the 0.04% also warm the other molecules by a bit as they are cooled (has to go both ways). ]]
However, there is also the law of ‘diminishing returns’- and the reason this is important is because these CO2 molecules re-absorb the same IR photons over and over and over again- The CO2 radiates it out- it’s neighbor absorbs it, it radiates it out and the original CO2 molecule reabsorbs it and so o nand so forth- until entropy causes it to fizzle out (the energy)
[[Since 0.04% of those molecules are CO2, there are 10^18 CO2 molecules of CO2 in just a liter of atmosphere. ]]
So are you saying there are 99.96% more non CO2 molecules per litre? If so this means we’re back again to massive percentage differences in CO2 and Non CO2 molecules- there just aren’t enough CO2 molecules to intercept and radiate heat or IR photons- we’re back to dumping a thimble of 102 degree water into an Olympic sized pool of 100 degree water and claiming it raised the temperature of the pool when clearly the mass volume of outside forces (the air surrounding the pool) is what regulates the temperature of the pool- The thimble, (or even 55 gallon drum if you like - not sure which one would represent 0.04% of the volume of the pool) of warmer
[[That’s a lot of molecules and a lot of chances to intercept an IR photon. The fact that it is such a low percentage (0.04%) is mitigated by the fact that air has so many molecules.]]
That can’t be- there MUST be vast swaths of atmosphere that have no CO2 molecules- an let’s not forget, even if there is a ‘blanket’ of CO2, one nano second the blanket is saturated, and in that nano second, IR photons blow right on past, so the same amount it absorbs, it also must let pass because I n that nano second, it can’t absorb another molecule- and also let’s not forget that the CO2 is reabsorbing molecules it has already absorbed, meaning new molecules go through at an even greater rate unimpeded
Let’s say there is a 1x1 foot layer of CO2- a thin layer- perhaps a molecule in thickness (I’m beign generous here by saying that the whole 1x1 foot area is covered with this layer)- now let’s assume there are, for the sake of illustration, 5 billion CO2 molecules in this layer. now let assume, for the sake of argument that 500 billion IR photons rise towards that layer and impact it all at once- the layer is going to become immediately saturated, and in that nano second, the same amount of photons that got absorbed will pass by without being absorbed because the layer is saturated, and is radiating energy out to be reabsorbed by it’s neighbor CO2 molecules etc-
Until the layer is able to ‘purge itself’ of all previously absorbed photon molecules, it won’;t be able to absorb new molecules, and so more molecules will blow on past than will ever be absorbed- it’s a numbers came it seems to me?
Note: I have no idea if any of this is even close to being correct- just trying to think through this logically, or as logically as my tired mind can muster-
You are trying to make the argument that because there is a ‘large amount’ that the exponentially larger amount of Non CO2 molecules doesn’t matter- It MUST matter-
Where is the evidence that CO2 captures all ir photons?
86
posted on
12/28/2015 10:06:19 PM PST
by
Bob434
To: palmer
[[1) the CO2 molecules get way warmer than 102F]]
What temperature is the released radiated energy?
87
posted on
12/28/2015 10:08:45 PM PST
by
Bob434
To: palmer
and just onem ore thought before my brain goes to shutdown-
the amount of Ir absorbed is still just 0.04% of the atmosphere- these get absorbed, radiated out in all directions- some upwards where they lose their energy, some sideways where they are reabsorbed and ‘perhaps’ lose some energy, and some downwards towards earth where the energy is met with lower temps in overwhelming numbers (IE there is vastly more cooler molecules than the fraction of the 0.04% radiated molecules that are warmer- These warmer molecules will quickly reach equilibrium with the earth’s temps being that the warmer molecule numbers are vastly outnumbered by the cooler earth surface molecules
The warmed molecules that remain in atmosphere (after being radiated sideways) will have a very slight effect on surrounding O2 molecules, but the warmer radiated molecules will also quickly reach equilibrium with atmospheric molecules/temps because once again the warmer molecules are vastly outnumbered by O2 molecules-
Again it comes down to sheer numbers- Sheer percentages- only a tiny fraction of the molecules captured by the scant CO2 (0.04% fo the atmosphere) will actually even remain in atmosphere- so while just 0.04% of the atmosphere is an insignificant amount, what remains I nthe atmosphere (the energy captured and released by the 0.04% cO2 molecules) is an even more insignificant percentage since only a fraction of that heat/energy actually remains in atmosphere
Again- by way of analogy, all we’re doing it pouring a 5 gallon bucket of slightly warmer water into an Olympic sized pool and claiming it will ‘cause catastrophic calamities if we don’t stop pouring a five gallon bucket in once a year’
88
posted on
12/28/2015 10:23:03 PM PST
by
Bob434
To: Texas Eagle
"It is not a fake. I remember this issue. I was in high school at the time and we discussed the topic in class." The cover is fake. The story is real, and was printed in the 24 June 1974 issue of Time on page 86. See it here: http://time.com/vault/issue/1974-06-24/page/106/
I remember it too, not because I read Time Magazine, but because it was on the evening news and we discussed it at school.
89
posted on
12/28/2015 10:56:42 PM PST
by
ETCM
To: Bob434
Until the layer is able to ‘purge itself’ of all previously absorbed photon molecules, it won’;t be able to absorb new molecules, and so more molecules will blow on past than will ever be absorbed- it’s a numbers came it seems to me? Yes, it is the saturation argument. Adding more CO2 to the atmosphere doesn't change the fact that all the outgoing photons are absorbed. But what changes is the photons are absorbed at a slight lower altitude.
Where is the evidence that CO2 captures all ir photons?
That was a mistake. It absorbs them all only within certain bands: http://www.atmos-chem-phys.net/13/6687/2013/acp-13-6687-2013.html That also lends support to the saturation argument.
90
posted on
12/29/2015 4:57:52 AM PST
by
palmer
(Net "neutrality" = Obama turning the internet over to foreign enemies)
To: Bob434
The 0.04% is significant because there are so many molecules and 0.04% of a lot is still a lot of molecules. The 0.028% that we started with 100 or so years ago is also a lot. Because of saturation adding the extra molecules to bump it up to 0.04% does not have a linear warming effect, but logarithmic.
91
posted on
12/29/2015 5:01:33 AM PST
by
palmer
(Net "neutrality" = Obama turning the internet over to foreign enemies)
To: Texas Eagle
BE WORRIED. BE VERY WORRIED. Don't worry, be happy.
92
posted on
12/29/2015 5:18:24 AM PST
by
ROCKLOBSTER
(Celebrate "Republicans Freed the Slaves Month")
To: palmer
The 0.04% is significant because there are so many molecules and 0.04% of a lot is still a lot of molecules. No, it isn't.
Calculate the molecules of making up the entire atmosphere and then strike off the zeros.
Besides, there is no "layer"
93
posted on
12/29/2015 5:30:27 AM PST
by
ROCKLOBSTER
(Celebrate "Republicans Freed the Slaves Month")
To: ROCKLOBSTER
The carbon cycle. Is that basics not even taught. Are ANY systems taught anymore. No, just one piece of the cycle.
Where is the biology discussion? where is that in the “model”
When I took botany the research showed plants were carbon starved. If we wanted to increase plant growth in a greenhouse, we pumped co2 in it because lack of co2 was a plant growth limiter. In fact, plants had a secondary high energy use photosynthetic mechanism to capture c02.
94
posted on
12/29/2015 6:10:54 AM PST
by
PeterPrinciple
(Thinking Caps are no longer being issued but there must be a warehouse full of them somewhere.)
To: Tennessean4Bush
The global average temperature has increased since the 1980's...The average global temperature for the last 10 years is approximately 0.35 degrees centigrade higher than it was during the 1980'sA real scientist would ask, first, "What is a "global average temperature", how do you measure it, and has the method of measurement changed between 1980 and 2015?
95
posted on
12/29/2015 6:15:23 AM PST
by
Jim Noble
(Diseases desperate grown Are by desperate appliance relieved Or not at al)
To: palmer
[[Adding more CO2 to the atmosphere doesn’t change the fact that all the outgoing photons are absorbed.]]
Do you have something which shows all are absorbed? You yourself said some slip by without beign absorbed
Also- do you have evidence that all are converted to energy?
An even if all are absorbed and converted to ehat/energy- it’s still only 0.04% of the total atmosphere that gets absorbed and converted, and the surrounding MASS of atmospheric O2 molecules at lower temps works to cool them to an equilibrium state almost immediately- one o2 molecule becomes slightly warmer by the process, but the molecule next to it is cooler- and so it cools the one molecule down as does the process of entropy
[[The 0.04% is significant because there are so many molecules and 0.04% of a lot is still a lot of molecules.]]
Not when you are talking the volume we’re talking about- heck, not when you are talking about any volume- 0.04% of any volume isn’t enough to affect anything- Take a glass of water- 100 degrees- drop in 0.04% 105 degree water- it does nothing because there isn’t volume enough of the hotter water to do anything
IF there were a thick blanket of CO2 that made up I dunno- 40% of the atmosphere, we could make the argument that heat can not get past it I suppose (although there would still be the issue of saturation)-
0.04% is NOT a lot- Especially given the fact that we are talking the volume of atmosphere that we are- it is still just 0.04% of the atmosphere-
What is the tonnage of IR photons converted to energy/heat? While the CO2 may be constantly converting small amounts of photons to energy/heat, you also have to factor In the fact that there is constant cooling going on in the atmosphere as well, and that the amount of cooling is still 99.95% greater than the amount of warming- there’s just no way around this - Just like if you dump 0.04% hotter water into an Olympic sized pool- The cooling and entropy from outside sources simply overwhelms any slight effect that adding the warmer water could have-
This is why I think the argument of LTE is so important because it is only small isolated areas that would ‘slightly’ be affected BRIEFLY by adding warmth
[[That was a mistake. It absorbs them all only within certain bands:]]
Bands of what? All GHT’s? Water vapor, Methane etc? Isn’t CO2 the only one capable of converting ir photons to energy/heat? Whereas the other GHG’s simply absorb and retain briefly?
96
posted on
12/29/2015 11:07:09 AM PST
by
Bob434
To: Bob434
Also- do you have evidence that all are converted to energy? If they strike a CO2 molecule, and they are the right frequency, they push that molecule into one of three higher energy states.
it's still only 0.04% of the total atmosphere that gets absorbed and converted,
It is 100% of the outgoing photons that are in the right frequency bands to be absorbed by CO2.
0.04% of any volume isn't enough to affect anything- Take a glass of water- 100 degrees- drop in 0.04% 105 degree water-
Drop in 102 degree water molecules at a 0.04% concentration into water at 100 degrees and you raise the entire water temperature from 100 to 100.001 Do it again one second later and you have 100.002, etc. The IR photons keep on coming and will warm the atmosphere as long as the surface or lower atmosphere is warmer than higher up.
Bands of what? All GHT's? Water vapor, Methane etc? Isn't CO2 the only one capable of converting ir photons to energy/heat? Whereas the other GHG's simply absorb and retain briefly?
Sorry, should have explained that. Frequency bands. They are pictured like this:
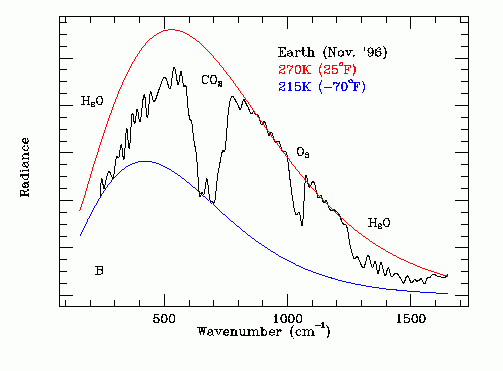
This is a view from space of IR emitted from the earth;s surface. The notches are frequencies where photons are being absorbed by various molecules in the atmosphere. The overall shape is due to the blackbody radiative spectrum of various earth surface temperatures, but the notches are caused by specific molecules. As you can see, O2 also creates a notch. I had completely forgotten that O2 and N2 are also "greenhouse gases", but that is a fact, explained in this paper: http://onlinelibrary.wiley.com/store/10.1029/2012GL051409/asset/grl29125.pdf?v=1&t=iirysn0v&s=cd95912488fed49f9ab101d20551181676ad1ef1
97
posted on
12/29/2015 2:29:56 PM PST
by
palmer
(Net "neutrality" = Obama turning the internet over to foreign enemies)
To: palmer
[[Drop in 102 degree water molecules at a 0.04% concentration into water at 100 degrees and you raise the entire water temperature from 100 to 100.001]]
no sir that can’t possibly be true- The mass of 100 degree water is far too great to allow that great a rise- and this also ignores the fact that the surrounding mass is not static but dynamic- what insignificant warming does happen is over-ruled by the mass of cooler upper level molecules- The amount of warming that takes place is far too small and the atmosphere too dynamic to remain around to create an accumulative effect- the principle I believe being that as air is warmed, it expands, (no matter how slightly) and then it rises, and cooler air replaces it (while at same time cooling the slightly warmer air)
In order for the atmosphere to remain warmer due to accumulative effect- it would need to somehow defeat the rising temps and sinking cooler temps, and it would need to be a % large enough to ‘overpower’ the mass of cooler air and dynamic environment
This cooling effect by convection far surpasses the slight warming effect due to CO2 and GHG- The accumulative effect you described is simply far too small to actually accumulate in an amount large enough to overcome the cooling effect- The cooling effect cancels out any warming- just like a room heated in isolation from outside environments- as soon as you open the doors, and allow cooler air in, the room cools, and will remain cool despite trying to warm it by adding 0.04% by volume heat to the room
Inm your closed system analogy of accumulation- We would have been at 100% warming long before now- but the fact is the system is not closed, and because it isn’t convection deals the slight warming a blow-
And don’t forget, them ore clouds/water vapor in the atmosphere (Due to upward heat act from earth to atmosphere) them ore solar radiation is reflected back out to space- Preventing that heat from warming the atmosphere like it would without cloud cover- The cooler the atmosphere due to lack of warming from space, the harder time the heated molecules created by CO2 has to cause brief localized warming
For the atmosphere to be hearted by CO2, there would need to be a ‘hotspot’ where heat is trapped and prevented from rising to the stratosphere- Guess what? There is no hotspot- none have been found- it’s a hypothesis which is being proven wrong- According to this report, the IPCC hypothesis is ‘right’ In theory, but wrong according to reality- but even if right according to reality- there is still so little warming that a hotspot would create that it would amount to nearly zero warming for the net atmosphere- and just slight warming for the localized hotspot-
http://sciencespeak.com/MissingSignature.pdf
This article says better than what I was trying to about entropy being devastating to heat accumulation in an open system- for the creation opf hotspots, the atmosphere would need to experience and imposible reduction of entropy:
[[From the mathematical definition of entropy, a process in which heat flows from cold to hot has decreasing entropy. This can happen in a non-isolated system if entropy is created elsewhere, such that the total entropy is constant or increasing.]]
http://hockeyschtick.blogspot.com/2010/07/why-agw-hot-spot-wont-happen.html
98
posted on
12/29/2015 3:34:41 PM PST
by
Bob434
To: Bob434
no sir that can't possibly be true- The mass of 100 degree water is far too great to allow that great a rise Assume there are 4 molecules at 102 degrees and 9996 molecules at 100 degrees, same ratio as CO2 to all other air molecules The average temperature of all molecules is (408 + 999600) / 10000 which is 100.0008 which I rounded up. But nearly every instant a CO2 molecule is heated so the temperature keeps rising until equilibrium. The tiny bit of warming cumulative (although subject to convection as you note)
the principle I believe being that as air is warmed, it expands, (no matter how slightly) and then it rises,
Yes and convection cools the atmosphere. The more convection the cooler it will get. But that just reduces massive warming by the sun and the little bit of warming by added CO2. Convection is a perfect example of a negative feedback. Negative feedbacks dominate otherwise the planet would have cooked long ago.
This cooling effect by convection far surpasses the slight warming effect due to CO2 and GHG-
Some places that is true. Other places it is not. The reason is that convection is weather and some places have subsidence (the opposite of convection) simply due to weather. CO2 heats evenly everywhere including convection locations and subsidence locations.
Inm your closed system analogy of accumulation- We would have been at 100% warming long before now- but the fact is the system is not closed, and because it isn't convection deals the slight warming a blow-
Yes, I have imagined what would happen with unstoppable CO2 warming and indeed convection and latent heat transfer limit the temperature rise. They also limit the far higher temperature rise resulting from daytime solar most places with sunshine.
For the atmosphere to be hearted by CO2, there would need to be a "hotspot" where heat is trapped and prevented from rising to the stratosphere- Guess what? There is no hotspot- none have been found- it's a hypothesis which is being proven wrong
No, the lack of a hotspot in the tropical troposphere just means models are wrong and can't predict water vapor feedback or cloud feedback or any other positive or negative feedbacks (if any) from the small amount of warming from added CO2. That small amount of warming from added CO2 is not something that comes from a climate model with errors like the hotspot. It comes from line by line models, essentially a simple formation of the graph I showed in my last comment. What the line by line models tell us backs up the actual radiance measurements shown in the graph. And that is that there are notches where some frequencies are being absorbed by CO2. Lots of frequencies absorbed by water vapor. And even a few frequencies absorbed by N2 and O2.
99
posted on
12/29/2015 4:46:13 PM PST
by
palmer
(Net "neutrality" = Obama turning the internet over to foreign enemies)
To: palmer
[[Assume there are 4 molecules at 102 degrees and 9996 molecules at 100 degrees]]
Where do you come up with 10,000? CO2 takes up just 0.04% of the atmosphere (forgive me but Math was never my strong point)
[[The average temperature of all molecules is (408 + 999600) / 10000 which is 100.0008]]
You are stating that those 4 molecules raise the temperature of all the 9996 molecules the same amount/degree %, but this can’t be as as soon as the energy/heat leaves the CO2 molecule, and transfers into neighbor molecule, it begins a rapid descent in temp as the molecule that received the energy/heat rises and become cooled-
The article I posted to shows that the mass of cooler molecules so vastly outweighs the warmed ones, and the impact of entropy is so pervasive, that the amount of IR caused warming is insignificant
[[The tiny bit of warming cumulative]]
I get that it’s accumulative, BUT it is as you say tiny- and as the other article I cited shows, the warmer molecuels rise, and are not caught in a closed system local ‘hot spot’ in the atmosphere
[[Some places that is true. Other places it is not.]]
The convection spoken of in the article I believe was speaking to upper cooler molecules descending down to the area of CO2- replacing the warmer with cooler molecules as the warmer ones rose- it wasn’t speaking to surface air conditions on earth- here you are talking about weather related changes which are local, not global, and have nothing to do with the small amount of atmospheric warming
[[CO2 heats evenly everywhere including convection locations and subsidence locations.]]
We’re not talking about the mechanism behind CO2 and heating- we’re talking about the fact that as soon as a molecule is heated it rises and is replaced by cooler descending molecules- and we’re talking about the fact that the upper cooler molecules vastly outnumber CO2 molecules-
[[And that is that there are notches where some frequencies are being absorbed by CO2. Lots of frequencies absorbed by water vapor. And even a few frequencies absorbed by N2 and O2]]
We know this occurs- that isn’t the issue- the issue is that the models are wrong about the impact because in order for the impact of the slight warming to do what the IPCC claims it will do in 100 years, there needs to be hotspots in an isolated closed system in order for the temps to reach what has been predicted— the whole alarmist predictions rely on this hot spot feature- I’m willing to bet your line by line models did not predict - The link to ‘line by line’ model was ‘forbidden’- does the line by lien model predict the future? Or is it simply recording present conditions- I have no idea what that graph you show even means- Graphs are also not a strong point for me-
[[ but the notches are caused by specific molecules. As you can see, O2 also creates a notch.]]
It seems to me the chart just shows that notches occure- not predict what will happen I nthe future- I can’t find any articles on frequency band models or lien by line models
[[The IR photons keep on coming and will warm the atmosphere as long as the surface or lower atmosphere is warmer than higher up.]]
They can keep coming all they want- but they are not accumulative in the atmosphere due to reasons cited- the stratosphere is many many times more volume than the atmosphere, and many times more than CO2 volume, and like the article I cited states, it simply overwhelms the tiny amount of warming- if it didn’t, the atmosphere would have been saturated with warmer molecules by now and we’d be cooked as you say- The amount of warming is nowhere near enough to change the temps anywhere near what the alarmists claim it will- there simply isn’t enough CO2 nor IR photons coming up or down to cause any kind of change except temporary local insignificant warming- You have just too many opposite effects going on for the slight warming to have any significant impact- Warming depends on suspending entropy and isolating the warming in a closed system globally, not just locally in spots around the globe
And by the way- NASA has just been caught fudging and hiding data, and the gauges used to determine temps around the globe have been exposed as beign biased to newly blacktopped areas, beside large heating and cooling motors on dark rooftops etc- These temp readings are a large part of any computer models
As well satellite data conflicts with computer models of any stripes- and I’m willing to bet that the pause has thrown a big monkey wrench into the equations as well-
Even Hansen has come out and said the paris climate deal is nonsense and will do nothing even if we stopped all our CO2 production globally- Hopefully Hansen will do the right thing and redeem himself by exposing this fraud for what it is
100
posted on
12/29/2015 10:57:28 PM PST
by
Bob434
Navigation: use the links below to view more comments.
first previous 1-20 ... 41-60, 61-80, 81-100, 101-110 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson
