Also- do you have evidence that all are converted to energy? If they strike a CO2 molecule, and they are the right frequency, they push that molecule into one of three higher energy states.
it's still only 0.04% of the total atmosphere that gets absorbed and converted,
It is 100% of the outgoing photons that are in the right frequency bands to be absorbed by CO2.
0.04% of any volume isn't enough to affect anything- Take a glass of water- 100 degrees- drop in 0.04% 105 degree water-
Drop in 102 degree water molecules at a 0.04% concentration into water at 100 degrees and you raise the entire water temperature from 100 to 100.001 Do it again one second later and you have 100.002, etc. The IR photons keep on coming and will warm the atmosphere as long as the surface or lower atmosphere is warmer than higher up.
Bands of what? All GHT's? Water vapor, Methane etc? Isn't CO2 the only one capable of converting ir photons to energy/heat? Whereas the other GHG's simply absorb and retain briefly?
Sorry, should have explained that. Frequency bands. They are pictured like this:
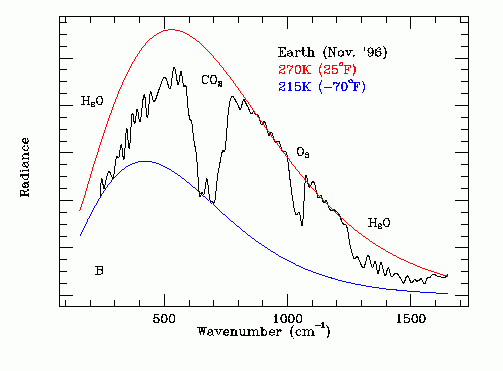
This is a view from space of IR emitted from the earth;s surface. The notches are frequencies where photons are being absorbed by various molecules in the atmosphere. The overall shape is due to the blackbody radiative spectrum of various earth surface temperatures, but the notches are caused by specific molecules. As you can see, O2 also creates a notch. I had completely forgotten that O2 and N2 are also "greenhouse gases", but that is a fact, explained in this paper: http://onlinelibrary.wiley.com/store/10.1029/2012GL051409/asset/grl29125.pdf?v=1&t=iirysn0v&s=cd95912488fed49f9ab101d20551181676ad1ef1
[[Drop in 102 degree water molecules at a 0.04% concentration into water at 100 degrees and you raise the entire water temperature from 100 to 100.001]]
no sir that can’t possibly be true- The mass of 100 degree water is far too great to allow that great a rise- and this also ignores the fact that the surrounding mass is not static but dynamic- what insignificant warming does happen is over-ruled by the mass of cooler upper level molecules- The amount of warming that takes place is far too small and the atmosphere too dynamic to remain around to create an accumulative effect- the principle I believe being that as air is warmed, it expands, (no matter how slightly) and then it rises, and cooler air replaces it (while at same time cooling the slightly warmer air)
In order for the atmosphere to remain warmer due to accumulative effect- it would need to somehow defeat the rising temps and sinking cooler temps, and it would need to be a % large enough to ‘overpower’ the mass of cooler air and dynamic environment
This cooling effect by convection far surpasses the slight warming effect due to CO2 and GHG- The accumulative effect you described is simply far too small to actually accumulate in an amount large enough to overcome the cooling effect- The cooling effect cancels out any warming- just like a room heated in isolation from outside environments- as soon as you open the doors, and allow cooler air in, the room cools, and will remain cool despite trying to warm it by adding 0.04% by volume heat to the room
Inm your closed system analogy of accumulation- We would have been at 100% warming long before now- but the fact is the system is not closed, and because it isn’t convection deals the slight warming a blow-
And don’t forget, them ore clouds/water vapor in the atmosphere (Due to upward heat act from earth to atmosphere) them ore solar radiation is reflected back out to space- Preventing that heat from warming the atmosphere like it would without cloud cover- The cooler the atmosphere due to lack of warming from space, the harder time the heated molecules created by CO2 has to cause brief localized warming
For the atmosphere to be hearted by CO2, there would need to be a ‘hotspot’ where heat is trapped and prevented from rising to the stratosphere- Guess what? There is no hotspot- none have been found- it’s a hypothesis which is being proven wrong- According to this report, the IPCC hypothesis is ‘right’ In theory, but wrong according to reality- but even if right according to reality- there is still so little warming that a hotspot would create that it would amount to nearly zero warming for the net atmosphere- and just slight warming for the localized hotspot-
http://sciencespeak.com/MissingSignature.pdf
This article says better than what I was trying to about entropy being devastating to heat accumulation in an open system- for the creation opf hotspots, the atmosphere would need to experience and imposible reduction of entropy:
[[From the mathematical definition of entropy, a process in which heat flows from cold to hot has decreasing entropy. This can happen in a non-isolated system if entropy is created elsewhere, such that the total entropy is constant or increasing.]]
http://hockeyschtick.blogspot.com/2010/07/why-agw-hot-spot-wont-happen.html
