Posted on 05/03/2010 10:50:42 PM PDT by neverdem
A new catalyst that generates hydrogen from sea water has been developed by scientists in the US. This new metal-oxo complex displays high catalytic activity and stability, whilst being low cost, the researchers say.
Hydrogen is very attractive as a clean source of power. Currently, it is produced by natural gas reforming - where steam is reacted with methane in the presence of a nickel catalyst to form hydrogen - but this method produces the greenhouse gas carbon dioxide.
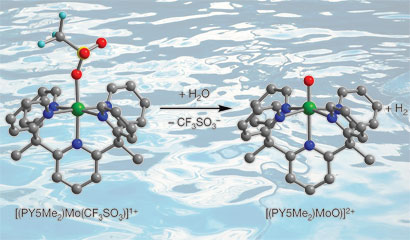
Jeffrey Long and colleagues from the University of California, Berkeley, prepared a simple molybdenum-oxo complex that can serve as an electrocatalyst, reducing the energy required to generate hydrogen from water on a mercury electrode. As an abundant metal, molybdenum is much cheaper than precious metal catalysts where the costs associated with large scale hydrogen production would be high.

|
The team's molybdenum-oxo species generates hydrogen from sea water
© Nature
|
Long explains that the stability of the catalyst is due to a ligand that bonds to the molybdenum in five places (pentadentate) making it a very strong complex. 'The molecule is very robust and is stable in aqueous conditions for long periods of time so we don't see degradation of the catalytic activity over three days of running the reaction,' he says.
Significantly, Long's catalyst is also stable in the presence of impurities that can be found in the ocean, meaning that sea water can be used without pre-treatment. The team used a sample of California sea water in the system and found the results to be similar to the results obtained for water at neutral pH. In addition, no other electrolyte is necessary when using sea water, which helps reduce costs and removes any need for organic acids or solvents that could degrade the catalyst.
'The work clearly demonstrates that the molybdenum-oxo complex explored shows good catalytic activity, with at least an order of magnitude higher turnover frequency [the speed at which a catalytic cycle is completed] than alternative catalysts quoted,' says Bruce Ewan, an expert in hydrogen production and renewable energy at the University of Sheffield, UK. 'This new catalyst also opens up new possibilities as a catalytic agent in other proton reducing scenarios,' he adds.
Long and his team hope to develop this system so that 'in the future a catalyst like this could be used in conjunction with a solar cell to produce hydrogen,' he explains. The team is now working on modifying the catalyst to reduce the potential at which the electrochemical reaction proceeds and make the system more efficient.
H I Karunadasa, C J Chang and J R Long, Nature, 2010, DOI: 10.1038/nature08969
Seawater is 1 ppm uranium...they’d be better off extracting uranium from seawater.
ping
And whence comes the energy to conduct this catalytic reaction? What becomes of the expended catalyst and is it toxic. Molydenum, what is the energy cost to produce that and is it not toxic to a degree????? Nothing without a price.
Another claiming to have circumvented the laws of thermodynamics?
Oops! You missed a step. When the hydrogen is consumed as fuel - a very good thing - water is formed in the atmosphere which precipitates as rain. This rain will eventually wind up in the oceans - they get refilled. We therefore have a virtually perpetual fuel source.
That's because they are progressives" - and therefore they cannot have an end point."Change" is their entire philosophy.
As with all hydrogen schemes the question as to distribution methods will rear it's head. Hydrogen is not an easy gas to handle or transport.
Use of the term "half-life" to describe ingredients in a system that is largely steady-state is very simplistic. Water simply changes state regularly, and there is no chemical alteration of it. CO2 is chemically altered when it is generated by burning fuel and is therefore not in the same class as water for your analogy.
.....tin foil....
Aluminum foil?
Finding the right catalyst will determine whether the electrolysis of water for using hydrogen as a fuel is economically viable or not, IMHO.
LOL! They actually said, “whilst?”
Nope. Producing is fine. H+ is simply a proton. H2 is a molecule. By “Hydrogen,” they mean H2, not protons. Water is not H2. It does not contain H2.
You don’t extract something unless it exists already in what you extract it from.
sorry,
reynolds wrap?
al
commonly called tin foil in the hinderlans were I grew up
ya, that can happen (kof kof) by accident
dry ice does the same thing is much safer
lye (drano) is dangerous if one is not careful 0
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.