Skip to comments.
'Artificial leaf' makes fuel from sunlight (w/ video)
http://www.physorg.com ^
| Sep 30, 2011
| by David L. Chandler
Posted on 09/30/2011 6:34:40 AM PDT by Red Badger
Researchers led by MIT professor Daniel Nocera have produced something they’re calling an “artificial leaf”: Like living leaves, the device can turn the energy of sunlight directly into a chemical fuel that can be stored and used later as an energy source.
The artificial leaf — a silicon solar cell with different catalytic materials bonded onto its two sides — needs no external wires or control circuits to operate. Simply placed in a container of water and exposed to sunlight, it quickly begins to generate streams of bubbles: oxygen bubbles from one side and hydrogen bubbles from the other. If placed in a container that has a barrier to separate the two sides, the two streams of bubbles can be collected and stored, and used later to deliver power: for example, by feeding them into a fuel cell that combines them once again into water while delivering an electric current.
The creation of the device is described in a paper published Sept. 30 in the journal Science. Nocera, the Henry Dreyfus Professor of Energy and professor of chemistry at MIT, is the senior author; the paper was co-authored by his former student Steven Reece PhD ’07 (who now works at Sun Catalytix, a company started by Nocera to commercialize his solar-energy inventions), along with five other researchers from Sun Catalytix and MIT.
The device, Nocera explains, is made entirely of earth-abundant, inexpensive materials — mostly silicon, cobalt and nickel — and works in ordinary water. Other attempts to produce devices that could use sunlight to split water have relied on corrosive solutions or on relatively rare and expensive materials such as platinum.
The artificial leaf is a thin sheet of semiconducting silicon — the material most solar cells are made of — which turns the energy of sunlight into a flow of wireless electricity within the sheet. Bound onto the silicon is a layer of a cobalt-based catalyst, which releases oxygen, a material whose potential for generating fuel from sunlight was discovered by Nocera and his co-authors in 2008. The other side of the silicon sheet is coated with a layer of a nickel-molybdenum-zinc alloy, which releases hydrogen from the water molecules.
“I think there’s going to be real opportunities for this idea,” Nocera says. “You can’t get more portable — you don’t need wires, it’s lightweight,” and it doesn’t require much in the way of additional equipment, other than a way of catching and storing the gases that bubble off. “You just drop it in a glass of water, and it starts splitting it,” he says.
Now that the “leaf” has been demonstrated, Nocera suggests one possible further development: tiny particles made of these materials that can split water molecules when placed in sunlight — making them more like photosynthetic algae than leaves. The advantage of that, he says, is that the small particles would have much more surface area exposed to sunlight and the water, allowing them to harness the sun’s energy more efficiently. (On the other hand, engineering a system to separate and collect the two gases would be more complicated in such a setup.)
The new device is not yet ready for commercial production, since systems to collect, store and use the gases remain to be developed. “It’s a step,” Nocera says. “It’s heading in the right direction.”
Ultimately, he sees a future in which individual homes could be equipped with solar-collection systems based on this principle: Panels on the roof could use sunlight to produce hydrogen and oxygen that would be stored in tanks, and then fed to a fuel cell whenever electricity is needed. Such systems, Nocera hopes, could be made simple and inexpensive enough so that they could be widely adopted throughout the world, including many areas that do not presently have access to reliable sources of electricity.
Professor James Barber, a biochemist from Imperial College London who was not involved in this research, says Nocera’s 2008 finding of the cobalt-based catalyst was a “major discovery,” and these latest findings “are equally as important, since now the water-splitting reaction is powered entirely by visible light using tightly coupled systems comparable with that used in natural photosynthesis. This is a major achievement, which is one more step toward developing cheap and robust technology to harvest solar energy as chemical fuel.”
Barber cautions that “there will be much work required to optimize the system, particularly in relation to the basic problem of efficiently using protons generated from the water-splitting reaction for hydrogen production.” But, he says, “there is no doubt that their achievement is a major breakthrough which will have a significant impact on the work of others dedicated to constructing light-driven catalytic systems to produce hydrogen and other solar fuels from water. This technology will advance side by side with new initiatives to improve and lower the cost of photovoltaics.”
Nocera’s ongoing research with the artificial leaf is directed toward “driving costs lower and lower,” he says, and looking at ways of improving the system’s efficiency. At present, the leaf can redirect about 2.5 percent of the energy of sunlight into hydrogen production in its wireless form; a variation using wires to connect the catalysts to the solar cell rather than bonding them together has attained 4.7 percent efficiency. (Typical commercial solar cells today have efficiencies of more than 10 percent). One question Nocera and his colleagues will be addressing is which of these configurations will be more efficient and cost-effective in the long run.
Another line of research is to explore the use of photovoltaic (solar cell) materials other than silicon — such as iron oxide, which might be even cheaper to produce. “It’s all about providing options for how you go about this,” Nocera says.
More information: http://www.science … 816.full.pdf
TOPICS: Business/Economy; Culture/Society; US: Massachusetts
KEYWORDS: electricity; energy; fuelcell; hydrogen
Navigation: use the links below to view more comments.
first 1-20, 21-26 next last

The 'artificial leaf,' a device that can harness sunlight to split water into hydrogen and oxygen without needing any external connections, is seen with some real leaves, which also convert the energy of sunlight directly into storable chemical form. Photo: Dominick Reuter
Video at link.
An 'artificial leaf' made by Daniel Nocera and his team, using a silicon solar cell with novel catalyst materials bonded to its two sides, is shown in a container of water with light (simulating sunlight) shining on it. The light generates a flow of electricity that causes the water molecules, with the help of the catalysts, to split into oxygen and hydrogen, which bubble up from the two surfaces.Video courtesy of the Nocera Lab/Sun Catalytix
To: Red Badger
To: Red Badger
Just add water, and this leaf is the size of the Hoover Dam.
To: Red Badger
4
posted on
09/30/2011 6:46:29 AM PDT
by
two23
(Liberals Have Created a Culture of Lies)
To: Red Badger
If successful, it’s just another energy source liberals will be out to kill. Hydrogen fuel produces no CO2, but it does produce water vapor, an even more effective green house gas.
5
posted on
09/30/2011 6:47:38 AM PDT
by
Telepathic Intruder
(The right thing is not always the popular thing)
To: Red Badger
Seems like every time something revolutionary like this comes along, you hear about it once or twice and then never again. We’ll see where this goes.
6
posted on
09/30/2011 6:48:51 AM PDT
by
RC one
(Voting isn't a simple act of civic duty anymore, it's a complex act of civil war.)
To: Red Badger
I don't "get" what is new about this
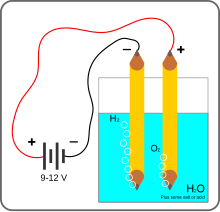
We used to use electrolysis back in high school to separate H2O into H and O. Solar cell produces electricity, electricity via electrolysis produces H and O. What is the big deal?
7
posted on
09/30/2011 7:01:31 AM PDT
by
jpsb
To: Red Badger
Isn’t it just a solar cell dunked in water - inefficiently using sunlight to create electricity, and then producing hydrogen at presumably the normal 40% efficiency?
I wonder how much energy is used to make this ‘leaf’.
8
posted on
09/30/2011 7:05:32 AM PDT
by
lacrew
(Mr. Soetoro, we regret to inform you that your race card is over the credit limit.)
To: jpsb
No batteries necessary. Sunlight only. Not even wires are necessary. Light, water and the device.................
9
posted on
09/30/2011 7:06:59 AM PDT
by
Red Badger
(We cannot defeat an enemy that the president and hence his administration cannot name.......)
To: lacrew
The device, Nocera explains, is made entirely of earth-abundant, inexpensive materials — mostly silicon, cobalt and nickel — and works in ordinary water. Other attempts to produce devices that could use sunlight to split water have relied on corrosive solutions or on relatively rare and expensive materials such as platinum.
10
posted on
09/30/2011 7:08:45 AM PDT
by
Red Badger
(We cannot defeat an enemy that the president and hence his administration cannot name.......)
To: Red Badger
Yeah free energy, sunlight, however the system H2O to H and O is still an inefficient use of energy even thou it is free energy. Sorry I am just not seeing this as a big deal, all that is going to happen is kids and handymen are going to blow themselves up.
11
posted on
09/30/2011 7:13:56 AM PDT
by
jpsb
To: jpsb
—What is the big deal?—
If I understand this, the big deal is twofold:
1. instead of a battery, it uses the sun.
2. it is made of cheap material.
12
posted on
09/30/2011 7:15:29 AM PDT
by
cuban leaf
(Were doomed! Details at eleven.)
To: Red Badger
Quick! Loan that leaf a half a billion and house it in a $700 mil facility with whistling robots!
To: Red Badger
I hope no bad guys get a hold of this and make huge leaves, then drop then into our water supplies!!!
14
posted on
09/30/2011 7:28:06 AM PDT
by
stuartcr
("Everything happens as God wants it to...otherwise, things would be different.")
To: Telepathic Intruder
I imagine that water vapor would then be used to replenish the leaf supply.
15
posted on
09/30/2011 7:29:40 AM PDT
by
stuartcr
("Everything happens as God wants it to...otherwise, things would be different.")
To: Red Badger
Here's the problem with this technology...
Bugs that depend on nutrition (possibly rare bugs, headed for extinction)from leaves, will be attracted to these phony leaves for food and will die of malnutrition. The horror, the mayhem, the absolute destruction of our sensitive ecosystem, will follow, the survival of the planet is in jeopardy, life as we no it is at stake.
The EPA must rescue us from this evil.
16
posted on
09/30/2011 7:42:49 AM PDT
by
PoloSec
( Believe how that Christ died for our sins, was buried and rose again for our justification)
To: Red Badger
The artificial leaf — a silicon solar cell with different catalytic materials bonded onto its two sides — needs no external wires or control circuits to operate. Simply placed in a container of water and exposed to sunlight, it quickly begins to generate streams of bubbles: oxygen bubbles from one side and hydrogen bubbles from the other. If placed in a container that has a barrier to separate the two sides, the two streams of bubbles can be collected and stored, and used later to deliver power: for example, by feeding them into a fuel cell that combines them once again into water while delivering an electric current. Neat trick... so how does the H and O2 know which side to form bubbles on when the molecule that is being split cannot be on both?
17
posted on
09/30/2011 8:31:14 AM PDT
by
BoneHead
To: jpsb
The problem with solar, wind, and most other alternate energy sources is that they are too diffuse - to power vehicles and machinery and such, one generally needs sources of concentrated energy.
Atomic nuclei are the best in terms of concentrated sources of energy that can be tapped, but nuclear reactors don’t fit well into cars and such.
Chemical bonds are the next best, so the real goal in the *practical* exploitation of solar (as opposed to the Obama exploitation of solar) is to take the energy of the sun and to store it in chemical bonds. That’s exactly what’s going on in this paper.
There are problems with handling O2 and H2, but there are problems with handling flammable volatile hydrocarbons as well (check out the gasoline fight in Zoolander!). Those problems can be solved, and if the O2 and H2 are used to drive a fuel cell, one comes up with a conceptually beautiful loop ofcollecting sunlight, using its energy, and in the process just taking water in one place and regenerating that water somewhere else.
This finding could really be a piece of a major development in energy technology.
18
posted on
09/30/2011 8:32:13 AM PDT
by
Stosh
To: BoneHead
It’s a atomic level shift of atoms among the molecules. The molecule loses its hydrogen atoms so it’s Oxygen atom immediately takes two from its neighbor. The chain reaction goes on all around the other side of the ‘leaf’ (the water is continuous), until a final oxygen atom has no neighbor to take from, so it exits the stage........
19
posted on
09/30/2011 8:37:38 AM PDT
by
Red Badger
(We cannot defeat an enemy that the president and hence his administration cannot name.......)
To: BoneHead
“...so how does the H and O2 know which side to form bubbles on when the molecule that is being split cannot be on both?”
I’m willing to bet there are different catalysts present on each side of the sheet. The catalysts on one side use the energy from the sun to take electrons away from the oxygens of water, forming the O2.
The catalysts attached to the *other face* of the sheet use the energy from the sun to give the electrons from the oxygen to the hydrogens, forming H2.
Each reaction happens on a different side of the sheet, so each gas forms on its own side (the electrons have to be able to cross the sheet, which apparently they can).
The real trick, I think, will be related to how many cycles you can get out of the catalysts before they degrade.
Kind of the same thing happens in an electrolysis process, with each gas forming at its own electrode - as described by a poster above, the problem being that to do the electrolysis, you have to use lots of energy from traditional sources.
20
posted on
09/30/2011 8:40:41 AM PDT
by
Stosh
Navigation: use the links below to view more comments.
first 1-20, 21-26 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson

