REVEALING OF ABORTION-BREAST CANCER LINK IS AIM OF SUIT AGAINST PLANNED PARENTHOOD
[Not News to FReepers:] First Case Linking Abortion-Breast Cancer Settled
WOMEN who have had an ABORTION TWICE as likely to BREAST CANCER
First ever Abortion-Breast Cancer Settlement [ABORTION causes BREAST CANCER]
British Scientists: Abortion Doubles Breast Cancer Risk
Abortion/breast cancer link can't be denied ^
| Breast Cancer: Risks and Prevention (Booklet) | |
| Single Most Avoidable Risk (Brochure) |
FACT SHEETS
Always a Bitter Pill, Now the Risk of Breast Cancer Makes Oral Contraceptives Even Harder to Swallow
"It may not have rocked the ground like the 1945 detonation of the first atomic bomb . . . but Enovid did more than just provide a technological tour de force. It transformed the very fabric of modern society . . ."
So reported "The Pill At 40", an article in the July-August 2000 "FDA Consumer" magazine, singing the praises of the Pill and celebrating the 40th anniversary of its approval by the Food and Drug Administration. On June 23, 1960, Enovid became the first oral contraceptive approved for sale in the USA, following several years of development and trials on third world women.
The article failed to mention the bitter legacy of the Pill over that same 40 years. Minor side effects abound, such as nausea, irregular bleeding, depression, weight gain, breast tenderness, and diminished libido. Some, however, are life threatening. Blood clots, pulmonary embolism, heart attack, and stroke have claimed the lives of many women taking the Pill since its introduction in 1960. Decreasing the dosages of the hormones in the Pill have lessened but not eliminated these deadly risks.
"Postfertilization Effects of Oral Contraceptives and Their Relationship to Informed Consent" was the first medical journal article to explain the mechanism by which the Pill prevents implantation of a fertilized egg in the womb, its lining (or endometrium) improperly formed under the influence of the Pill's hormones. Published in the February 2000 Archives of Family Medicine, a journal of the American Medical Association, it proved for both the secular world and a divided pro-life movement that the Pill is not only a contraceptive but also a chemical abortifacient. The report concludes:
"The available evidence supports the hypothesis that when ovulation and fertilization occur in women taking OCs, postfertilization effects are operative on occasion to prevent clinically recognized pregnancy. Physicians should understand and respect the beliefs of patients who consider human life to be present and valuable from the moment of fertilization."
While litigation in the USA relative to the Pill has been limited to suits aimed at forcing insurance plans to cover the Pill, in Britain a class action lawsuit has begun addressing another aspect of informed consent. In January 2002, 122 women and/or their families will take three pharmaceutical companies before England's High Court, charging that the Pill has caused blood clots resulting in lifelong illnesses and even death, and that they were never informed of the severe risks. Ten percent of the 122 claims involve a fatality. Unfortunately, these side effects have been known for four decades, and the prospects of success for these victims are uncertain.
However, compelling data has emerged linking the Pill with the rapid increase of breast cancer in the US, with a potential of class action lawsuits that could eclipse even those of the tobacco industry. Evidence has been available for several decades linking oral contraceptives with breast cancer in certain lab animals. According to Chris Kahlenborn, MD, one of the nation's leading researchers on the breast cancer/ Pill connection, the evidence of a link in humans is incontrovertible. His book summarizing his research and findings, BREAST CANCER: Its Link to Abortion and the Birth Control Pill, was published recently by One More Soul (www.OMS.com.) Dr. Kahlenborn has testified before the FDA regarding the issues in his book, and the book's findings are now part of the federal record.
In the book he makes a compelling case for such a link. He began researching the issue after hearing a presentation in 1993 that described an increase in breast cancer risk due to abortion, apparently caused by hormonal changes in the woman's body. He began an exhaustive review of the research to ascertain whether contraceptive hormones in the Pill might have the same effect.
When asked, "What is the bottom-line, after 8 years of exhaustive research and study?" Dr. Kahlenborn replied, "There is a 45% increased risk of developing breast cancer if a woman takes an oral contraceptive prior to her first full term pregnancy. This number is statistically significant to the 99th percentile."
"Informed consent is MIA. Catholic OB/GYN's are doing a grave disservice in handing this out. Today's cigarette story [the tobacco class action lawsuits] could be tomorrow's Pill story. There is no informed consent. The breast cancer and the social effects cause such devastation to families!"
He compares the current state of denial among the American medical establishment to a similar episode that occurred several decades ago. "History is repeating itself. DES was taken in the 40's and 50's to prevent miscarriage. A 35% increased risk of breast cancer was found." At the time DES (diethylstilbestrol) was used, some were concerned of a potential risk of breast cancer, while the American medical establishment denied the possibility. Only after 25 years was it discovered that DES use carried a 35% increased breast cancer risk.
Currently, more than 192,000 U.S. women develop breast cancer and more than 40,000 die from it each year. One in eight women in the US will be diagnosed with breast cancer in their lifetime. Yet 50 years ago, breast cancer was less common. When asked what other factors might account for such a rapid increase in the rates of breast cancer, Dr. Kahlenborn was blunt. "I am not sure of all the factors but two other factors come into play: decreased family size and decreased breast-feeding. Pregnancy and breast-feeding have been known to protect against breast cancer for many years. But the Pill and abortion also are likely responsible for the rising rates of breast cancer in Western countries." Breast cancer is increasing more rapidly in western countries, countries with early Pill use, often in the teen years, usually before first full term pregnancy.
Medical research findings have been contradictory. In 1972 a series of animal research studies showed that an oral contraceptive appeared to cause metastatic breast cancer in rhesus monkeys, which rarely develop breast cancer. In 1989 Anderson et al published a paper that found that women who had never had children who took the Pill had a significantly higher rate of breast cell division than childless mothers who had never taken the Pill. In general, cells that divide more rapidly are more vulnerable to carcinogens and more likely to become cancerous. A study in 1981 found that women who took the Pill for 4 years prior to their first full-term pregnancy (FFTP) had a 125% increased risk of breast cancer before age 32. In 1993, the CASH study showed a 40% increased risk in women taking the Pill before FFTP. Later in England another large study revealed a 44% increased risk. The last large study in 1995 showed a 42% increased risk. A meta-analysis (a statistical analysis of many other research studies) in 1990 found that, overall, the studies up to that time confirmed an increased risk of breast cancer of 72% for women under age 45 who took oral contraceptive pills for 4 or more years before having a full-term pregnancy. Use of these contraceptives for longer periods appears to carry an even higher risk.
However, the Oxford study, the largest meta-analysis to date, concluded that:
"Women who are currently using [the Pill] or have used them in the past 10 years are at a slightly increased risk of having breast cancer diagnosed, although the additional cancers tend to be localized to the breast. There is no evidence of an increase in the risk of having breast cancer diagnosed 10 or more years after cessation of use..."
Dr. Kahlenborn sees severe weaknesses in the Oxford study. He states in his book:
"The main weakness was the failure to report any evidence of what the pooled risk of oral contraceptive use before a FFTP was in women under 45 years old . . . A woman's breast is especially sensitive to carcinogenic influence . . . before [FFTP] because the breast undergoes a maturing process throughout a woman's first pregnancy. By failing to measure the effect . . . before a . . . woman's [FFTP] the Oxford study failed to give data on the one group of women who are most likely to get breast cancer from oral contraceptives." Dr. Kahlenborn commented further, "In addition the Oxford meta-analysis used studies whose data came from as early as the 1960s. This is not precise enough since these studies would not have picked up the Pill's effects on the breast (ie, too short of a latent period).
Currently Dr. Kahlenborn is working on another meta-analysis that he hopes will be published within one year. This analysis attempts to analyze the data of all the studies available from the 1980's and 1990's, in an effort to obtain a more accurate statistical analysis specifically of women taking the Pill for several years prior to their first full-term pregnancy.
The Food and Drug Administration's FDA consumer magazine maintained that Enovid may not have rocked the ground like the 1945 detonation of the first atomic bomb. Dr. Kahlenborn would be inclined to disagree. "Hormonal chemical contraceptives are the equivalent to a nuclear bomb in their devastation to the family." Sickness, cancer and death lies in the wake of this bitter Pill. Can massive product liability suits be far behind?
"Neglecting to inform women of the link between abortion and breast cancer could mean thousands of them pay the price with their lives." Concerned Women for America
![]() Coalition on Abortion and Breast Cancer
Coalition on Abortion and Breast Cancer![]() Real Statistics on Abortion and Breast Cancer
Real Statistics on Abortion and Breast Cancer![]() The Breast Cancer Connection
The Breast Cancer Connection![]() CWA Summary on Abortion-Breast Cancer
CWA Summary on Abortion-Breast Cancer![]() 1998 Wisconsin Law Review on Disclosure Duty
1998 Wisconsin Law Review on Disclosure Duty![]() Abortion Increases the Risk of Breast Cancer
Abortion Increases the Risk of Breast Cancer![]() Abortion-Breast Cancer Studies, 1981-1996
Abortion-Breast Cancer Studies, 1981-1996![]() Politics of Breast Cancer Research
Politics of Breast Cancer Research![]() Why the Silence About Abortion and Breast Cancer: Chicago Tribune; May 21, 2001
Why the Silence About Abortion and Breast Cancer: Chicago Tribune; May 21, 2001![]() Abortion & Breast Cancer FAQ-Chris Kahlenborn, MD
Abortion & Breast Cancer FAQ-Chris Kahlenborn, MD![]() Link between Abortion and Breast Cancer
Link between Abortion and Breast Cancer![]() Dr. Janet Daling on Abortion-Breast Cancer
Dr. Janet Daling on Abortion-Breast Cancer![]() Additional Evidence of Abortion-Cancer: J. Brind, Ph.d.
Additional Evidence of Abortion-Cancer: J. Brind, Ph.d.![]() World Conference on Cancer: July 1997
World Conference on Cancer: July 1997![]() Testimony before Food & Drug Administration: July 1996
Testimony before Food & Drug Administration: July 1996![]() Additional Breast Cancer Studies: S. Summerville
Additional Breast Cancer Studies: S. Summerville![]() Return to Teen Pregnancy Home Page
Return to Teen Pregnancy Home Page
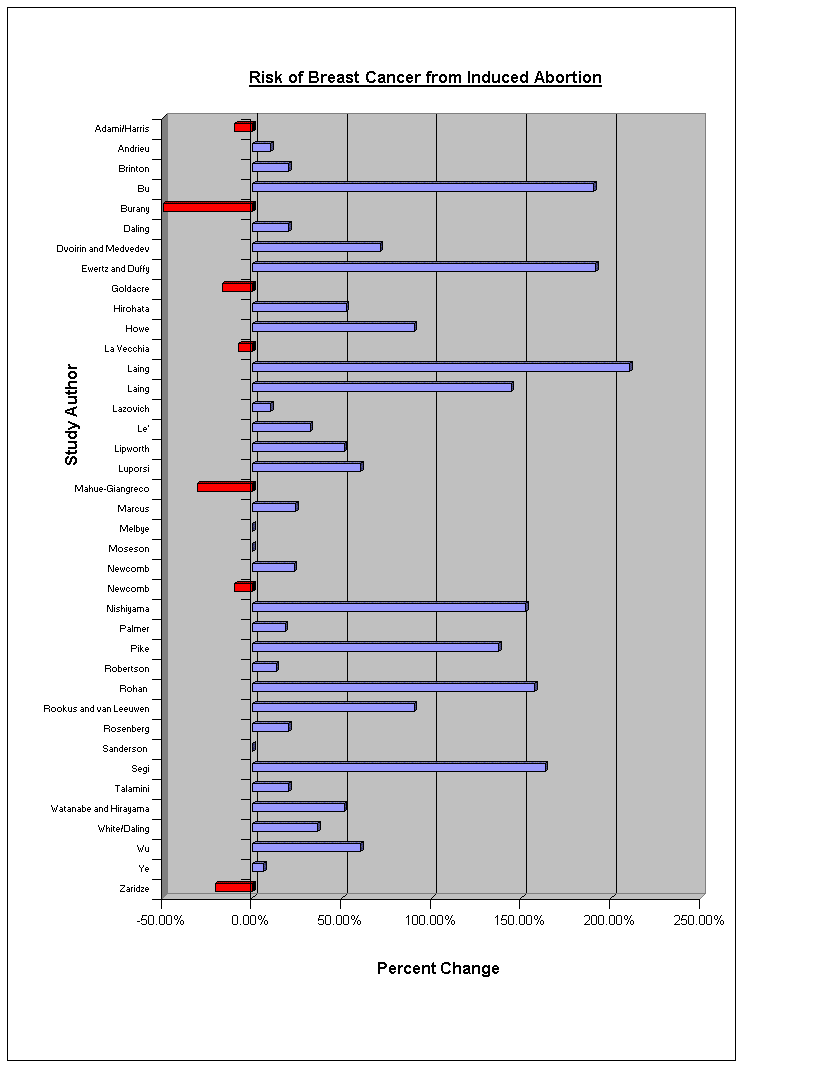
Abortion and Breast Cancer Statistics
Dr. Janet Daling is a cancer researcher at the Fred Hutchinson Cancer Research Center and the University of Washington. Dr. Daling is self-described as 'pro-choice'. On 2 November 1994 Dr. Daling and fellow researchers published an article in the Journal of the National Cancer Institute (pp. 1584-1592) concerning induced abortion and breast cancer risk for premenopausal women. Some key findings:
- Women under age 18 who had an induced abortion have an increased breast cancer risk of 150%.
- Women of age 30 and above who aborted a first pregnancy increase their breast cancer risk by 110%.
- Overall, women who have an induced abortion have an increased breast cancer risk of 50%.
The Journal of the National Medical Association is a publication by black medical professionals concerned with black health problems. In the December 1993 issue JNMA published the results of a Howard University study. Key finding:
Black women of age 50 and above who had at least 1 induced abortion have an increased breast cancer risk of 370%.
Mike Richmond
Cancer Awareness Canada
810 W. Broadway (651)
Vancouver, Canada V5Z 4C9