Mechanochemical separation of gases using ball milling - Deakin University
MORE INFO:
https://www.sciencedirect.com/science/article/abs/pii/S1369702122001614?dgcid=author#f0025

Mechanochemical separation of gases using ball milling - Deakin University
MORE INFO:
https://www.sciencedirect.com/science/article/abs/pii/S1369702122001614?dgcid=author#f0025
Ping!...........
So it takes steel balls to make this stuff?
Nanoparticles have incredibly high surface-to-volume ratio.
I know a guy who makes nanoparticles with a few hundred to a few thousand atoms (or molecules) each out of all kinds of materials, by the tens of pounds.
When you open one of his shipping jars, it appears to be full of smoke. You don’t open one without PPE and under a fume hood.
Headline is a lie.
How much powder volume of powder with trapped hydrogen gas is needed for one liter of liquid hydrogen?
So the Hindenburg would be filled with hydrogen powder instead?
As a hiker/backpacker type, I’m waiting for powdered water ... something lighter than 8 lbs a gallon.
Something to figure out if there are cost effective applications for powdered hydrogen.
Definitely not a food additive, but maybe it’s also possible to powder other gasses with this method.
That’s what is used to make nanoparticles.
So in theory this would solve the problem of storing and transporting hydrogen for vehicles and what not. Correct?

You can pack a bunch of Hydrogen into a glass of water. The problem is that it has already spent it’s chemical energy bonding with Oxygen.
You put this powder up your nose you will literally do BLOW!
Isn’t that just a fancy name for Cocaine?
Kind of like Dehydrated Water.
This may be a breakthrough for energy storage and transportation, but what is the source of the energy required to power the gas separation process? And how much energy does this whole process require compared to the energy stored in the powdered hydrogen?
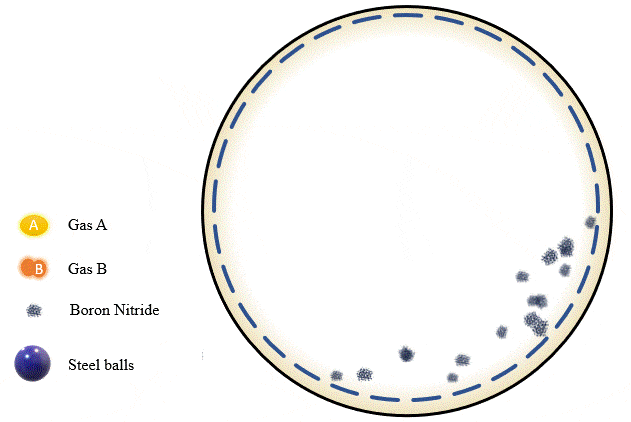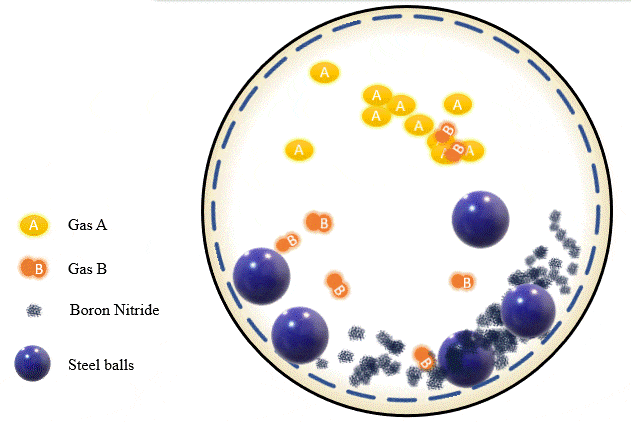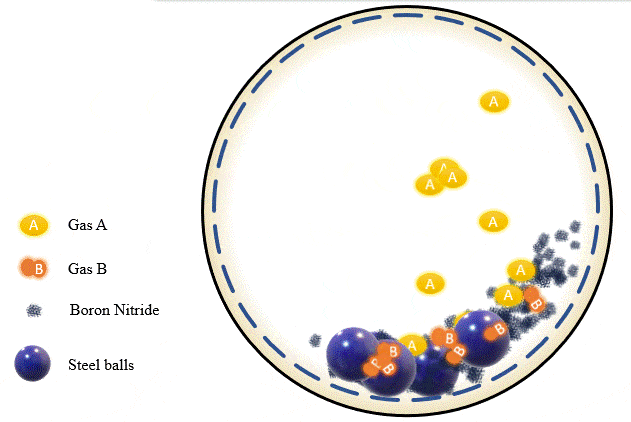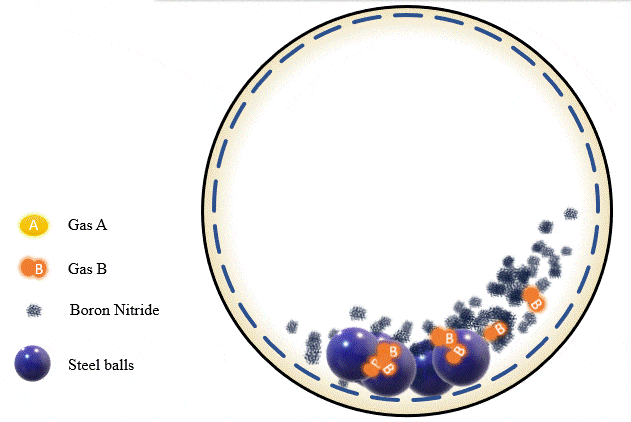
The team has demonstrated that grinding certain amounts of certain powders with precise pressure levels of certain gases can trigger a mechanochemical reaction that absorbs the gas into the powder and stores it there, giving you what’s essentially a solid-state storage medium that can hold the gases safely at room temperature until they’re needed. The gases can be released as required, by heating the powder up to a certain point.
—
Certainly are a lot of certains in that paragraph.
Powdered hydrogen.
Sounds dangerous.
Don’t let humper biden near that pile of white powder.
I would not snort that. Unless I was Hunter Biden?
“making hydrogen much easier and safer to store and transport in a powder”
Why do I foresee something (like excess humidity or the like) causing these powders to “leak” gas and cause a catastrophic detonation?