Silicon chip with porous surface next to the special furnace where it was coated with graphene to create a supercapacitor electrode. Credit: Joe Howell / Vanderbilt
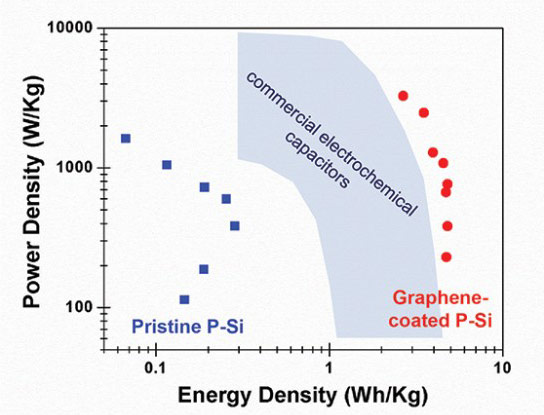
Graph displays the power density (watts per kilogram) and energy density (watt-hours per kilogram) of capacitors made from porous silicon (P-Si), graphene-coated porous silicon and carbon-based commercial capacitors. (Cary Pint / Vanderbilt)
To: ShadowAce
2 posted on
10/24/2013 1:12:29 PM PDT by
Red Badger
(The only way to defeat liberalism is to give them everything they want......then pick up the pieces.)
To: Red Badger
I wonder what it could do if you attached it to a DeLorean.
4 posted on
10/24/2013 1:18:24 PM PDT by
noiseman
(The only thing necessary for the triumph of evil is for good men to do nothing.)
To: Red Badger
material scientists? Whats that? A scientist that studies
material?
6 posted on
10/24/2013 1:20:45 PM PDT by
Slambat
To: Red Badger
That will make your flashlight recoil.
To: Red Badger
“improved energy densities by over two orders of magnitude compared to those made from uncoated porous silicon and significantly better than commercial supercapacitors.”
Cool! Big jump!
To: Red Badger
This is a continuation of the work pioneered by Rice and UCLA (the latter who discovered how to cheaply produce graphene) and shows great promise. The biggest problem I see is the safety factor of using high storage capacitors. The ability of capacitors to charge quickly also allows them to discharge quickly so a shorted capacitor basically explodes.
To: Red Badger
The graph shows a power density of around 4 W-h/kg. Lithium-ion batteries are 100–265 W·h/kg.
15 posted on
10/24/2013 1:47:49 PM PDT by
PapaBear3625
(You don't notice it's a police state until the police come for you.)
To: Red Badger
What is the comparison between the best super-capacitors and this?
18 posted on
10/24/2013 1:51:57 PM PDT by
sr4402
To: rdb3; Calvinist_Dark_Lord; Salo; JosephW; Only1choice____Freedom; amigatec; Still Thinking; ...
19 posted on
10/24/2013 1:52:35 PM PDT by
ShadowAce
(Linux -- The Ultimate Windows Service Pack)
To: Red Badger
They move through the graphene as a wave. It’s a wave! The moment to applaud would be now.
To: Red Badger
That graph shows grapheme caps topping out at about 4.5 Wh/Kg energy density.
Just for perspective, the best lithium polymer batteries deliver about 250 Wh/Kg.
The power density for supercaps is pretty good though, as is their longevity.
28 posted on
10/24/2013 3:20:10 PM PDT by
Steely Tom
(If the Constitution can be a living document, I guess a corporation can be a person.)
To: Red Badger
Will Dr Pint name the consortium to exploit this nanotech “Pint-sized materials”?
29 posted on
10/24/2013 5:01:02 PM PDT by
NonValueAdded
(Occupy the DC Mall - take back the monuments)
To: Red Badger
Graphene - like transistors were in the 50’s....
35 posted on
10/25/2013 7:32:27 AM PDT by
GOPJ
(Self-respect is the root of discipline...dignity grows with the ability to say no to oneself-Heschel)
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson
![]()
