Posted on 04/05/2011 6:48:10 AM PDT by blam
San Francisco Rainwater Radiation 181 Times Above US Drinking Water Standard
Politics / Environmental Issues
Apr 04, 2011 - 04:15 PM
By: DK Matai
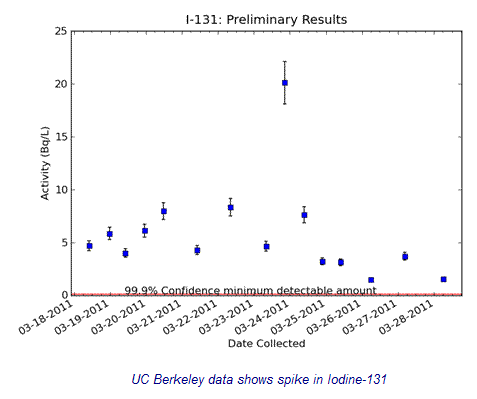
Radiation from Japan rained on Berkeley, California, during recent storms at levels that exceeded drinking water standards by 181 times. A rooftop water monitoring program managed by the University of California at Berkeley’s Department of Nuclear Engineering detected substantial spikes in rain-borne iodine-131 during those torrential downpours. The levels exceeded federal drinking water thresholds, known as Maximum Contaminant Levels -- or MCLs -- by as much as 181 times or 18,100%. Iodine-131 is one of the most cancer-causing toxic radioactive isotopes spewed when nuclear power plants are in meltdown. It is being ingested by cows, which have begun passing it through into their milk and radioactivity has been detected. [Multiple Sources]

Specific Scientific Data
The iodine-131 level in the rainwater sample taken on the roof of Etcheverry Hall on the campus of UC Berkeley on March 23rd, 2011, from 9:06-18:00hrs Pacific Daylight Time (PDT) states radioactivity levels at 20.1 Becquerels per Litre (Bq/L) = 543 PicoCuries per Litre (pCi/L). The federal maximum level of iodine-131 allowed in drinking water is 3 pCi/L or 0.111 Becquerels per Litre. The sample exceeded the federal guidelines for drinking water by 181 times. The UC Berkeley researchers also discovered trace levels of iodine-131 and other radioactive isotopes, believed to have originated in Fukushima, in commercially available milk and in a local stream within California. [UC Berkeley]
No Official Data Yet
Three weeks after the Fukushima nuclear power plant began spewing radiation into the world’s air, the US government has still not published any official data on nuclear fallout from the Fukushima meltdown. The amount of iodine-131 or other radioactive elements that have fallen as precipitation or made their way into milk supplies or drinking water has not yet been fully revealed. Scientists say an absence of federal data on the issue is hampering efforts to develop strategies for preventing radioactive isotopes from contaminating the nation's food and water. [The Bay Citizen, San Francisco]
Rising Risks
Fukushima radiation is blanketing most of the United States and Canada according to the data and visuals published regularly by the The Norwegian Institute of Air Research. The risks of that radiation falling with rain, have been downplayed by US government officials and others, who say its impacts are so fleeting and minor so as to be negligible. Nonetheless, radiation falling with rain can cover grass that is eaten by cows and other animals. It can also fall on food crops or contaminate reservoirs that are used for irrigation or drinking water. [Norwegian Institute of Air Research or NILU]
Food and Water Watch
Food and Water Watch -- the nonprofit Non-Governmental Organisation (NGO) based in Washington, DC -- sent a letter to President Barack Obama and members of his cabinet and Congress a few days ago urging the US federal government to improve its monitoring of radiation in agricultural land and food in the wake of the Japanese tragedy. The letter from "Food and Water Watch" states: “The three agencies that monitor almost all of the food Americans eat … have insisted that the US food supply is safe . . . the agencies, however, have done very little to detail specific ways in which they are responding to the threat of radiation in food.”
EPA and FDA
The federal Environmental Protection Agency (EPA) states in its April 3rd advisory, "As the Nuclear Regulatory Commission has said, we do not expect to see radiation at harmful levels reaching the US from damaged Japanese nuclear power plants." The US Food and Drug Administration (FDA), which regulates food safety, has referred questions about potential milk contamination to the EPA, which is taking the lead on testing dairy products for radiation. Early last week, the EPA said it expected to release results of tests for radioactivity in rain and snow within a day or so. Just before the weekend, three days after making that pledge, EPA officials repeated the same statement and said the data would likely be released over the weekend or early this week. So far that data set has not been released. [EPA]
Conclusion
Potentially cancer-causing radiation from Fukushima has been encircling the world, traveling quickly on jet streams high in the atmosphere and falling with precipitation like rain and snow. It is already being detected in air, water and milk in some parts of the United States by local and state agencies. For example, San Francisco rain water radiation levels exceeded federal drinking water thresholds by as much as 181 times recently. A radioactive isotope, such as iodine-131, is supposed to have a half-life of eight days. This is inferred to mean that it breaks down quickly, and it quickly dissipates in the environment. However, the 8 day half-life can be a misnomer because radioactive iodine can really persist in the environment for many months and has a 100 day biological half-life once inside the human body.
Right. And 2 + 2 is supposed to be 5. The halflives of isotopes is well characterized.
I grew up in the 1950s when the US was still doing atmospheric nuclear tests in Nevada and I’m sure many in my generation absorbed as much or more radiation in our milk without killing us off.
Berkely?....no problem.....they’ve all got two heads anyway.
And besides, 100x of a very, very, very tiny thing is still a very, very, very tiny thing.
Let’s say I know with absolute certainty that I will die if I ingest...
1.000000000000000
units of a certain toxin.
Let’s say that we have some evidence that in a population of a million persons, that a few hundred will get sick and a small handful will die if all million ingest...
0.000000000000001
units of this toxin.
How well can we predict the illness and death rates if the population of one million is exposed to AN ALARMING 1000 TIMES the rate of toxin? Or
0.000000000001000
units of this toxin.
Maybe not.
Milk goes from the cow to your store shelf in two to three days.
I-131 has a half-life of 8 days. Three half-lives in the general rule of thumb for an isotope to be decayed to a safe level from its initial level. So for I-131 that would be 24 days.
What the article does not say is that most milk cows in the US are fed on stored grain and hay which would not be exposed to rain fall.
So unless you are a person that drinks only organic pastured cow milk I would not be too worried.
My milk glows in the dark and I can use it as a night light.
islamism is still the greatest threat to us all.
Iodine 131 has a half-life of 8.03 days. If it truly has 181x the allowable level, keep it in your refigerator for two months and it will have decayed to permissible levels.
;o)
I agree....Anything to divert attention from the real threat.
Let me put 20 Bq in perspective: You get 15 Bq from eating a banana.
So that’s why those California cows are happy!! They glow in the dark so they can graze all night.
Looks like Wisconsin dairy is still the best. :^)
Let me put 20 Bq in perspective: You get 15 Bq from eating a banana.
Media types love to use “this many times” or “this percent above/below” on pretty much anything because it makes amore compelling headline than the raw numbers. For example (taken from “Bad Science” by Ben Goldacre) if a study showed that eating a certain food or taking a certain medicine increased the number of heart attacks per ten thousand men from 2 to 4, the headlines would scream that said food or medicine doubles your heart attack risk or is a 100% increase. Technically true, but not as dire as it sounds. The bummer is that when they use the same technique ( “Bran reduces your cancer risk by 50%!!!!!!”) it convinces people there’s a bulletproof solution to diseases.
Excellent point.
I’ve been looking all over the EPA website trying to find that particular drinking water standard. The closest I can find is a standard for gross beta, at 4 mrem/year.
I’m starting to believe the standard is not an official one, perhaps even made up.
I agree 100%.
Our children and grandchildren will one day ask why we didn't do something while we still could.
Excellent point. You’ll never get a job as “journalist” if you keep up the sensible talk, you know.
Well, there’s also this:
These guys are saying there’s an activity of 20 Bq per liter.
Eating a banana involves 15 Bq. So, already we have no risk.
But then consider that unless all the Iodine 131 in a liter of water goes straight to the milk with none staying in the cow, in any of the equipment, etc., the Bq is going to be reduced further by the time the product reaches you.
They are using the term 181 times because it makes a shocking headline. There’s nothing here.
http://water.epa.gov/drink/contaminants/index.cfm#Radionuclides
This does not mention I-131 but has standards for activity in general. It needs to be converted from pCi/l to Bq/l, to match the article, I’m quite busy so I’ll leave the conversion up to FReeperdom.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.