Posted on 02/08/2005 3:50:43 AM PST by PatrickHenry
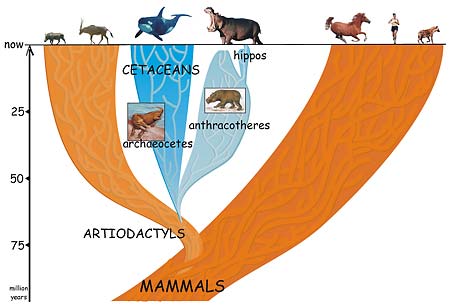
A group of four-footed mammals that flourished worldwide for 40 million years and then died out in the ice ages is the missing link between the whale and its not-so-obvious nearest relative, the hippopotamus.
The conclusion by University of California, Berkeley, post-doctoral fellow Jean-Renaud Boisserie and his French colleagues finally puts to rest the long-standing notion that the hippo is actually related to the pig or to its close relative, the South American peccary. In doing so, the finding reconciles the fossil record with the 20-year-old claim that molecular evidence points to the whale as the closest relative of the hippo.
"The problem with hippos is, if you look at the general shape of the animal it could be related to horses, as the ancient Greeks thought, or pigs, as modern scientists thought, while molecular phylogeny shows a close relationship with whales," said Boisserie. "But cetaceans – whales, porpoises and dolphins – don't look anything like hippos. There is a 40-million-year gap between fossils of early cetaceans and early hippos."
In a paper appearing this week in the Online Early Edition of the Proceedings of the National Academy of Sciences, Boisserie and colleagues Michel Brunet and Fabrice Lihoreau fill in this gap by proposing that whales and hippos had a common water-loving ancestor 50 to 60 million years ago that evolved and split into two groups: the early cetaceans, which eventually spurned land altogether and became totally aquatic; and a large and diverse group of four-legged beasts called anthracotheres. The pig-like anthracotheres, which blossomed over a 40-million-year period into at least 37 distinct genera on all continents except Oceania and South America, died out less than 2 and a half million years ago, leaving only one descendent: the hippopotamus.
This proposal places whales squarely within the large group of cloven-hoofed mammals (even-toed ungulates) known collectively as the Artiodactyla – the group that includes cows, pigs, sheep, antelopes, camels, giraffes and most of the large land animals. Rather than separating whales from the rest of the mammals, the new study supports a 1997 proposal to place the legless whales and dolphins together with the cloven-hoofed mammals in a group named Cetartiodactyla.
"Our study shows that these groups are not as unrelated as thought by morphologists," Boisserie said, referring to scientists who classify organisms based on their physical characteristics or morphology. "Cetaceans are artiodactyls, but very derived artiodactyls."

The origin of hippos has been debated vociferously for nearly 200 years, ever since the animals were rediscovered by pioneering French paleontologist Georges Cuvier and others. Their conclusion that hippos are closely related to pigs and peccaries was based primarily on their interpretation of the ridges on the molars of these species, Boisserie said.
"In this particular case, you can't really rely on the dentition, however," Boisserie said. "Teeth are the best preserved and most numerous fossils, and analysis of teeth is very important in paleontology, but they are subject to lots of environmental processes and can quickly adapt to the outside world. So, most characteristics are not dependable indications of relationships between major groups of mammals. Teeth are not as reliable as people thought."
As scientists found more fossils of early hippos and anthracotheres, a competing hypothesis roiled the waters: that hippos are descendents of the anthracotheres.
All this was thrown into disarray in 1985 when UC Berkeley's Vincent Sarich, a pioneer of the field of molecular evolution and now a professor emeritus of anthropology, analyzed blood proteins and saw a close relationship between hippos and whales. A subsequent analysis of mitochondrial, nuclear and ribosomal DNA only solidified this relationship.
Though most biologists now agree that whales and hippos are first cousins, they continue to clash over how whales and hippos are related, and where they belong within the even-toed ungulates, the artiodactyls. A major roadblock to linking whales with hippos was the lack of any fossils that appeared intermediate between the two. In fact, it was a bit embarrassing for paleontologists because the claimed link between the two would mean that one of the major radiations of mammals – the one that led to cetaceans, which represent the most successful re-adaptation to life in water – had an origin deeply nested within the artiodactyls, and that morphologists had failed to recognize it.
This new analysis finally brings the fossil evidence into accord with the molecular data, showing that whales and hippos indeed are one another's closest relatives.
"This work provides another important step for the reconciliation between molecular- and morphology-based phylogenies, and indicates new tracks for research on emergence of cetaceans," Boisserie said.
Boisserie became a hippo specialist while digging with Brunet for early human ancestors in the African republic of Chad. Most hominid fossils earlier than about 2 million years ago are found in association with hippo fossils, implying that they lived in the same biotopes and that hippos later became a source of food for our distant ancestors. Hippos first developed in Africa 16 million years ago and exploded in number around 8 million years ago, Boisserie said.
Now a post-doctoral fellow in the Human Evolution Research Center run by integrative biology professor Tim White at UC Berkeley, Boisserie decided to attempt a resolution of the conflict between the molecular data and the fossil record. New whale fossils discovered in Pakistan in 2001, some of which have limb characteristics similar to artiodactyls, drew a more certain link between whales and artiodactyls. Boisserie and his colleagues conducted a phylogenetic analysis of new and previous hippo, whale and anthracothere fossils and were able to argue persuasively that anthracotheres are the missing link between hippos and cetaceans.
While the common ancestor of cetaceans and anthracotheres probably wasn't fully aquatic, it likely lived around water, he said. And while many anthracotheres appear to have been adapted to life in water, all of the youngest fossils of anthracotheres, hippos and cetaceans are aquatic or semi-aquatic.
"Our study is the most complete to date, including lots of different taxa and a lot of new characteristics," Boisserie said. "Our results are very robust and a good alternative to our findings is still to be formulated."
Brunet is associated with the Laboratoire de Géobiologie, Biochronologie et Paléontologie Humaine at the Université de Poitiers and with the Collège de France in Paris. Lihoreau is a post-doctoral fellow in the Département de Paléontologie of the Université de N'Djaména in Chad.
The work was supported in part by the Mission Paléoanthropologique Franco-Tchadienne, which is co-directed by Brunet and Patrick Vignaud of the Université de Poitiers, and in part by funds to Boisserie from the Fondation Fyssen, the French Ministère des Affaires Etrangères and the National Science Foundation's Revealing Hominid Origins Initiative, which is co-directed by Tim White and Clark Howell of UC Berkeley.
Name one.
I understand you are a biologist, now could you please name two different species that are actually scientifically proven to have a common ancestor? It might help to name the common ancestor as well and indicate where I might find this proof.
"b. Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc2) is small but significant in nuclear reactions."
Above: Correct but misleading.
"Energy and mass equivalence
E= MC2
Exception” to E=EC2 (conservation) rule - only holds nuclear reactions sun (fusion), nuclear bomb, nuclear power; not related to combustion or day-to-day situations!"
Wrong!
Have you considered the question that I posed to you in post #1729?
Ha Ha Ha Ha ......
Perhaps you have the same position as Jaysun
I wasn't aware that this was how the subject of mass/energy equivalence was being taught. Personally, when I taught chemistry, I just talked about energy when dealing with chemical reactions and didn't bring up mass-energy equivalence until late in the year when we discussed nuclear reactions. At that time, I pointed out that all energy-producing reactions, chemical and nuclear, dervied their energy from mass conversion. I always thought that there wasn't any point in bringing the concept up during a discussion of chemical reactions, but I never would have intentionally miseducated students like that. I would guess that this is probably a result of poorly educated teachers who don't properly understand their subject matter.
Exactly.
I am sure I disagree in many ways with Jayson as well. In fact, if someone just tries to debate facts here you immediately get lumped in with one side or the other. The fact of the matter is that both polar sides are ignorant at this time (there are not enough facts to prove or disprove many of the theories). Why not continue to seek the truth (as in sceientific fact) rather than cry when someone point out a inconsistancy with a theory?
Also, I can not see why these debates get so emotional. Does anyone actually think that Christianity is in trouble if evolution proves to be true? I will tell you this, my faith is not so fragile.
One has to take J in context. Here was his first post on this thread:
"I bet the first whale that jumped on the beach and suddenly started breathing air only did so to get away from all of his pals and their cruel jokes about his freakish half hippo appearance."
And you expect us to take him seriously?
You misrepresent Shubi's post. He was not responding to a reasonable question, but responding to the rantings of Jaysun. Please be more careful in the future.
-------------------------------------------------------
[Jaysun]"I don't want to argue this at length. I don't subscribe to your idea and you don't subscribe to mine. We're even."
[Shubi]No we are not even. I am a biologist and you are not. I am a Biblical scholar and you are not.
1. M Nei and J Zhang, Evolution: molecular origin of species. Science 282: 1428-1429, Nov. 20, 1998. Primary article is: CT Ting, SC Tsaur, ML We, and CE Wu, A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282: 1501-1504, Nov. 20, 1998. As the title implies, has found the genes that actually change during reproductive isolation.
2. M Turelli, The causes of Haldane's rule. Science 282: 889-891, Oct.30, 1998. Haldane's rule describes a phase every population goes thru during speciation: production of inviable and sterile hybrids. Haldane's rule states "When in the F1 [first generation] offspring of two different animal races one sex is absent, rare, or sterile, that sex is the heterozygous [heterogemetic; XY, XO, or ZW] sex."Two leading explanations are fast-male and dominance. Both get supported. X-linked incompatibilities would affect heterozygous gender more because only one gene."
3. Barton, N. H., J. S. Jones and J. Mallet. 1988. No barriers to speciation. Nature. 336:13-14.
4. Baum, D. 1992. Phylogenetic species concepts. Trends in Ecology and Evolution. 7:1-3.
5. Rice, W. R. 1985. Disruptive selection on habitat preference and the evolution of reproductive isolation: an exploratory experiment. Evolution. 39:645-646.
6. Ringo, J., D. Wood, R. Rockwell, and H. Dowse. 1989. An experiment testing two hypotheses of speciation. The American Naturalist. 126:642-661.
7. Schluter, D. and L. M. Nagel. 1995. Parallel speciation by natural selection. American Naturalist. 146:292-301.
8. Callaghan, C. A. 1987. Instances of observed speciation. The American Biology Teacher. 49:3436.
9. Cracraft, J. 1989. Speciation and its ontology: the empirical consequences of alternative species concepts for understanding patterns and processes of differentiation. In Otte, E. and J. A. Endler [eds.] Speciation and its consequences. Sinauer Associates, Sunderland, MA. pp. 28-59.
10. Callaghan, C. A. 1987. Instances of observed speciation. The American Biology
Teacher. 49:3436.
Speciation in Insects
1. G Kilias, SN Alahiotis, and M Pelecanos. A multifactorial genetic investigation of speciation theory using drosophila melanogaster Evolution 34:730-737, 1980. Got new species of fruit flies in the lab after 5 years on different diets and temperatures. Also confirmation of natural selection in the process. Lots of references to other studies that saw speciation.
2. JM Thoday, Disruptive selection. Proc. Royal Soc. London B. 182: 109-143, 1972.
Lots of references in this one to other speciation.
3. KF Koopman, Natural selection for reproductive isolation between Drosophila pseudobscura and Drosophila persimilis. Evolution 4: 135-148, 1950. Using artificial mixed poulations of D. pseudoobscura and D. persimilis, it has been possible to show,over a period of several generations, a very rapid increase in the amount of reproductive isolation between the species as a result of natural selection.
4. LE Hurd and RM Eisenberg, Divergent selection for geotactic response and evolution of reproductive isolation in sympatric and allopatric populations of houseflies. American Naturalist 109: 353-358, 1975.
5. Coyne, Jerry A. Orr, H. Allen. Patterns of speciation in Drosophila. Evolution. V43. P362(20) March, 1989.
6. Dobzhansky and Pavlovsky, 1957 An incipient species of Drosophila, Nature 23: 289- 292.
7. Ahearn, J. N. 1980. Evolution of behavioral reproductive isolation in a laboratory stock of Drosophila silvestris. Experientia. 36:63-64.
8. 10. Breeuwer, J. A. J. and J. H. Werren. 1990. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature. 346:558-560.
9. Powell, J. R. 1978. The founder-flush speciation theory: an experimental approach. Evolution. 32:465-474.
10. Dodd, D. M. B. and J. R. Powell. 1985. Founder-flush speciation: an update of experimental results with Drosophila. Evolution 39:1388-1392. 37. Dobzhansky, T. 1951. Genetics and the origin of species (3rd edition). Columbia University Press, New York.
11. Dobzhansky, T. and O. Pavlovsky. 1971. Experimentally created incipient species of Drosophila. Nature. 230:289-292.
12. Dobzhansky, T. 1972. Species of Drosophila: new excitement in an old field. Science. 177:664-669.
13. Dodd, D. M. B. 1989. Reproductive isolation as a consequence of adaptive divergence in Drosophila melanogaster. Evolution 43:1308-1311.
14. de Oliveira, A. K. and A. R. Cordeiro. 1980. Adaptation of Drosophila willistoni experimental populations to extreme pH medium. II. Development of incipient reproductive isolation. Heredity. 44:123-130.15. 29. Rice, W. R. and G. W. Salt. 1988. Speciation via disruptive selection on habitat preference: experimental evidence. The American Naturalist. 131:911-917.
30. Rice, W. R. and G. W. Salt. 1990. The evolution of reproductive isolation as a correlated character under sympatric conditions: experimental evidence. Evolution. 44:1140-1152.
31. del Solar, E. 1966. Sexual isolation caused by selection for positive and negative phototaxis and geotaxis in Drosophila pseudoobscura. Proceedings of the National Academy of Sciences (US). 56:484-487.
32. Weinberg, J. R., V. R. Starczak and P. Jora. 1992. Evidence for rapid speciation following a founder event in the laboratory. Evolution. 46:1214-1220.
33. V Morell, Earth's unbounded beetlemania explained. Science 281:501-503, July 24, 1998. Evolution explains the 330,000 odd beetlespecies. Exploitation of newly evolved flowering plants.
34. B Wuethrich, Speciation: Mexican pairs show geography's role. Science 285: 1190, Aug. 20, 1999. Discusses allopatric speciation. Debate with ecological speciation on which is most prevalent.
Speciation in Plants
1. Speciation in action Science 72:700-701, 1996 A great laboratory study of the evolution of a hybrid plant species. Scientists did it in the lab, but the genetic data says it happened the same way in nature.
2. Hybrid speciation in peonies http://www.pnas.org/cgi/content/full/061288698v1#B1
3. http://www.holysmoke.org/new-species.htm new species of groundsel by hybridization
4. Butters, F. K. 1941. Hybrid Woodsias in Minnesota. Amer. Fern. J. 31:15-21.
5. Butters, F. K. and R. M. Tryon, jr. 1948. A fertile mutant of a Woodsia hybrid. American Journal of Botany. 35:138.
6. Toxic Tailings and Tolerant Grass by RE Cook in Natural History, 90(3): 28-38, 1981 discusses selection pressure of grasses growing on mine tailings that are rich in toxic heavy metals. "When wind borne pollen carrying nontolerant genes crosses the border [between prairie and tailings] and fertilizes the gametes of tolerant females, the resultant offspring show a range of tolerances. The movement of genes from the pasture to the mine would, therefore, tend to dilute the tolerance level of seedlings. Only fully tolerant individuals survive to reproduce, however. This selective mortality, which eliminates variants, counteracts the dilution and molds a toatally tolerant population. The pasture and mine populations evolve distinctive adaptations because selective factors are dominant over the homogenizing influence of foreign genes."
7. Clausen, J., D. D. Keck and W. M. Hiesey. 1945. Experimental studies on the nature of species. II. Plant evolution through amphiploidy and autoploidy, with examples from the Madiinae. Carnegie Institute Washington Publication, 564:1-174.
8. Cronquist, A. 1988. The evolution and classification of flowering plants (2nd edition). The New York Botanical Garden, Bronx, NY.
9. P. H. Raven, R. F. Evert, S. E. Eichorn, Biology of Plants (Worth, New York,ed. 6, 1999).
10. M. Ownbey, Am. J. Bot. 37, 487 (1950).
11. M. Ownbey and G. D. McCollum, Am. J. Bot. 40, 788 (1953).
12. S. J. Novak, D. E. Soltis, P. S. Soltis, Am. J. Bot. 78, 1586 (1991).
13. P. S. Soltis, G. M. Plunkett, S. J. Novak, D. E. Soltis, Am. J. Bot. 82,1329 (1995).
14. Digby, L. 1912. The cytology of Primula kewensis and of other related Primula hybrids. Ann. Bot. 26:357-388.
15. Owenby, M. 1950. Natural hybridization and amphiploidy in the genus Tragopogon. Am. J. Bot. 37:487-499.
16. Pasterniani, E. 1969. Selection for reproductive isolation between two populations of maize, Zea mays L. Evolution. 23:534-547.
Speciation in microorganisms
1. Canine parovirus, a lethal disease of dogs, evolved from feline parovirus in the 1970s.
2. Budd, A. F. and B. D. Mishler. 1990. Species and evolution in clonal organisms -- a summary and discussion. Systematic Botany 15:166-171.
3. Bullini, L. and G. Nascetti. 1990. Speciation by hybridization in phasmids and other insects. Canadian Journal of Zoology. 68:1747-1760.
4. Boraas, M. E. 1983. Predator induced evolution in chemostat culture. EOS. Transactions of the American Geophysical Union. 64:1102.
5. Brock, T. D. and M. T. Madigan. 1988. Biology of Microorganisms (5th edition). Prentice Hall, Englewood, NJ.
6. Castenholz, R. W. 1992. Species usage, concept, and evolution in the cyanobacteria (blue-green algae). Journal of Phycology 28:737-745.
7. Boraas, M. E. The speciation of algal clusters by flagellate predation. EOS. Transactions of the American Geophysical Union. 64:1102.
8. Castenholz, R. W. 1992. Speciation, usage, concept, and evolution in the cyanobacteria (blue-green algae). Journal of Phycology 28:737-745.
9. Shikano, S., L. S. Luckinbill and Y. Kurihara. 1990. Changes of traits in a bacterial population associated with protozoal predation. Microbial Ecology. 20:75-84.
New Genus
1. Muntzig, A, Triticale Results and Problems, Parey, Berlin, 1979. Describes whole new *genus* of plants, Triticosecale, of several species, formed by artificial selection. These plants are important in agriculture.
Invertebrate not insect
1. ME Heliberg, DP Balch, K Roy, Climate-driven range expansion and morphological evolution in a marine gastropod. Science 292: 1707-1710, June1, 2001. Documents mrorphological change due to disruptive selection over time. Northerna and southern populations of A spirata off California from Pleistocene to present.
2. Weinberg, J. R., V. R. Starczak and P. Jora. 1992. Evidence for rapid speciation following a founder event with a polychaete worm. . Evolution. 46:1214-1220.
Vertebrate Speciation
1. N Barton Ecology: the rapid origin of reproductive isolation Science 290:462-463, Oct. 20, 2000. www.sciencemag.org/cgi/content/full/290/5491/462 Natural selection of reproductive isolation observed in two cases. Full papers are: AP Hendry, JK Wenburg, P Bentzen, EC Volk, TP Quinn, Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science 290: 516-519, Oct. 20, 2000. and M Higgie, S Chenoweth, MWBlows, Natural selection and the reinforcement of mate recognition. Science290: 519-521, Oct. 20, 2000
2. G Vogel, African elephant species splits in two. Science 293: 1414, Aug. 24, 2001. http://www.sciencemag.org/cgi/conte...l/293/5534/1414
3. C Vila` , P Savolainen, JE. Maldonado, IR. Amorim, JE. Rice, RL. Honeycutt, KA. Crandall, JLundeberg, RK. Wayne, Multiple and Ancient Origins of the Domestic Dog Science 276: 1687-1689, 13 JUNE 1997. Dogs no longer one species but 4 according to the genetics. http://www.idir.net/~wolf2dog/wayne1.htm
4. Barrowclough, George F.. Speciation and Geographic Variation in Black-tailed Gnatcatchers. (book reviews) The Condor. V94. P555(2) May, 1992
5. Kluger, Jeffrey. Go fish. Rapid fish speciation in African lakes. Discover. V13. P18(1) March, 1992.
Formation of five new species of cichlid fishes which formed since they were isolated from the parent stock, Lake Nagubago. (These fish have complex mating rituals and different coloration.) See also Mayr, E., 1970. _Populations, Species, and Evolution_, Massachusetts, Harvard University Press. p. 348
6. Genus _Rattus_ currently consists of 137 species [1,2] and is known to have
originally developed in Indonesia and Malaysia during and prior to the Middle
Ages[3].
[1] T. Yosida. Cytogenetics of the Black Rat. University Park Press, Baltimore, 1980.
[2] D. Morris. The Mammals. Hodder and Stoughton, London, 1965.
[3] G. H. H. Tate. "Some Muridae of the Indo-Australian region," Bull. Amer. Museum Nat. Hist. 72: 501-728, 1963.
7. Stanley, S., 1979. _Macroevolution: Pattern and Process_, San Francisco,
W.H. Freeman and Company. p. 41
Rapid speciation of the Faeroe Island house mouse, which occurred in less than 250 years after man brought the creature to the island.
Science doesn't deal in proofs.
Try to rephrase your question in scientific terms. Besides the list I just posted has speciations, which necessarily
come from a "common ancestor".
Your problem in understanding science is based on misunderstandings of the definitions and methods science uses.
I posted 1729.
You are adhering to the first rule of propaganda, "when you are unable to answer a question, throw a lot of data at the question and claim that it is an answer."
I answered your question. Darwin's Finches are an example of multiple speciations from a single mother species.
You can't win at this game. And from now on, look it up yourself. Because you do not want to research, does not mean the information does not exist.
No. You said "Name ONE". If you don't like his "data dump" just pick the first ONE.
Can these diverse finches still mate with one another?
Biologists have generally learned about the divergence of species by comparing many different species at various stages of speciation. But in their study of the greenish warbler, a songbird that breeds in forests throughout much of temperate Asia, Irwin and his colleagues—Trevor D. Price, a biology professor at UCSD, and Staffan Bensch, a former postdoctoral student at UCSD now at Sweden’s Lund University—discovered a rare situation known to biologists as a "ring species."
"Ring species are unique because they present all levels of variation, from small differences between neighboring populations to species-level differences, in a single group of organisms,"
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.