Posted on 05/03/2010 10:50:42 PM PDT by neverdem
A new catalyst that generates hydrogen from sea water has been developed by scientists in the US. This new metal-oxo complex displays high catalytic activity and stability, whilst being low cost, the researchers say.
Hydrogen is very attractive as a clean source of power. Currently, it is produced by natural gas reforming - where steam is reacted with methane in the presence of a nickel catalyst to form hydrogen - but this method produces the greenhouse gas carbon dioxide.
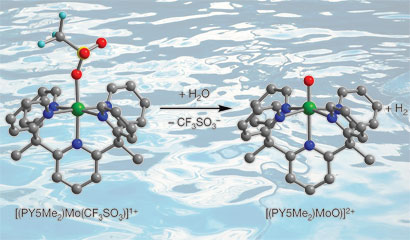
Jeffrey Long and colleagues from the University of California, Berkeley, prepared a simple molybdenum-oxo complex that can serve as an electrocatalyst, reducing the energy required to generate hydrogen from water on a mercury electrode. As an abundant metal, molybdenum is much cheaper than precious metal catalysts where the costs associated with large scale hydrogen production would be high.

|
The team's molybdenum-oxo species generates hydrogen from sea water
© Nature
|
Long explains that the stability of the catalyst is due to a ligand that bonds to the molybdenum in five places (pentadentate) making it a very strong complex. 'The molecule is very robust and is stable in aqueous conditions for long periods of time so we don't see degradation of the catalytic activity over three days of running the reaction,' he says.
Significantly, Long's catalyst is also stable in the presence of impurities that can be found in the ocean, meaning that sea water can be used without pre-treatment. The team used a sample of California sea water in the system and found the results to be similar to the results obtained for water at neutral pH. In addition, no other electrolyte is necessary when using sea water, which helps reduce costs and removes any need for organic acids or solvents that could degrade the catalyst.
'The work clearly demonstrates that the molybdenum-oxo complex explored shows good catalytic activity, with at least an order of magnitude higher turnover frequency [the speed at which a catalytic cycle is completed] than alternative catalysts quoted,' says Bruce Ewan, an expert in hydrogen production and renewable energy at the University of Sheffield, UK. 'This new catalyst also opens up new possibilities as a catalytic agent in other proton reducing scenarios,' he adds.
Long and his team hope to develop this system so that 'in the future a catalyst like this could be used in conjunction with a solar cell to produce hydrogen,' he explains. The team is now working on modifying the catalyst to reduce the potential at which the electrochemical reaction proceeds and make the system more efficient.
H I Karunadasa, C J Chang and J R Long, Nature, 2010, DOI: 10.1038/nature08969
You’re pretty much right from what I can remember about chemistry. Except there is no extra electron in Na+ to strip out... you’re short an electron!
The amount of energy needed to seperate a sizeable chunk of Sodium from all its valence-shell electrons would be staggering. But you could do this on a molecular level. That’s sorta what a catalyst is.
Oh yeah that’s right. If the Na+ is positive—that means it lacks an electron. So you’d have to add an electron. That looks harder than stripping out the extra electron.
lOL.
If this technology works, it will be a great thing. I was making fun of global warming nuts, whose goal is destruction of modern society, and control of our lives, not protection of the environment. You watch, if all our cars run on hydrogen and only water is the byproduct, you will see them come up with a theory that makes that a bad thing. Cars give people freedom; that threatens socialists.
You would spend more energy obtaining sodium ions than you would recover in the reaction. Most of the good primary chemical energy sources on the earth have already reacted with something and expended their reaction energy. The nuclear furnace 93 million miles away bombards us with electromagnetic energy which enterprising plants convert to chemical energy. We exploit the resulting plant energy as firewood, peat, coal and petroleum.
It is only because of plants that there is any free oxygen in the atmosphere, it would quickly have bonded with some other element otherwise.
You would spend more energy obtaining sodium ions than you would recover in the reaction.
.........
the sodium ions Na+ are already present in solution in saltwater.
the question is —thanks to the previous poster— where do you get the extra free electron from to attach to the sodium ion Na+ and how do you attach it in situ in solution so that suddenly a sodium metal Na is in the exothermic presence of water H2O
Way back then sodium was easily obtained and we didn't have much of a problem when we used it. I imagine now we would be classified as terrorists by having any on hand.
Correct, Na+ is the reaction product. If you dissolve a cup of salt in a quart of water, there will considerable heating, just from the salt going into solution. To recover metallic sodium from that solution requires more energy than recovering it from salt alone.
Significantly, Long's catalyst is also stable in the presence of impurities that can be found in the ocean, meaning that sea water can be used without pre-treatment. The team used a sample of California sea water in the system and found the results to be similar to the results obtained for water at neutral pH.The luddites will be out in force to get a seawater electrolysis ban. Thanks neverdem.
To recover metallic sodium from that solution requires more energy than recovering it from salt alone.
‘’’’’’’
But the point here is not to recover the the Na but rather to change Na+ to Na while its in solution with H20 —or rather as its settling out of solution with H20 which would cause an exothermic reaction.
I agree with you on both counts.
The sodium in salt water is matched with chloride atoms. It takes energy to separate them, more energy then you would get by reacting the sodium. Just remember the Second Law of Thermodynamics: “There ain’t no free lunch”, or something like that.
Whilst I have hitherto not seen the adverb “whilst” used in a modern document, I shall henceforth endeavor to make use of said term.
Prevalent, yes. Powerful, no.
Technically speaking molecule for molecule you are correct. My point is that water vapour overall is the most ‘powerful’ GHG in the atmosphere.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.