Posted on 05/03/2010 10:50:42 PM PDT by neverdem
A new catalyst that generates hydrogen from sea water has been developed by scientists in the US. This new metal-oxo complex displays high catalytic activity and stability, whilst being low cost, the researchers say.
Hydrogen is very attractive as a clean source of power. Currently, it is produced by natural gas reforming - where steam is reacted with methane in the presence of a nickel catalyst to form hydrogen - but this method produces the greenhouse gas carbon dioxide.
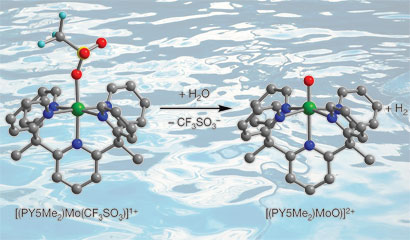
Jeffrey Long and colleagues from the University of California, Berkeley, prepared a simple molybdenum-oxo complex that can serve as an electrocatalyst, reducing the energy required to generate hydrogen from water on a mercury electrode. As an abundant metal, molybdenum is much cheaper than precious metal catalysts where the costs associated with large scale hydrogen production would be high.

|
The team's molybdenum-oxo species generates hydrogen from sea water
© Nature
|
Long explains that the stability of the catalyst is due to a ligand that bonds to the molybdenum in five places (pentadentate) making it a very strong complex. 'The molecule is very robust and is stable in aqueous conditions for long periods of time so we don't see degradation of the catalytic activity over three days of running the reaction,' he says.
Significantly, Long's catalyst is also stable in the presence of impurities that can be found in the ocean, meaning that sea water can be used without pre-treatment. The team used a sample of California sea water in the system and found the results to be similar to the results obtained for water at neutral pH. In addition, no other electrolyte is necessary when using sea water, which helps reduce costs and removes any need for organic acids or solvents that could degrade the catalyst.
'The work clearly demonstrates that the molybdenum-oxo complex explored shows good catalytic activity, with at least an order of magnitude higher turnover frequency [the speed at which a catalytic cycle is completed] than alternative catalysts quoted,' says Bruce Ewan, an expert in hydrogen production and renewable energy at the University of Sheffield, UK. 'This new catalyst also opens up new possibilities as a catalytic agent in other proton reducing scenarios,' he adds.
Long and his team hope to develop this system so that 'in the future a catalyst like this could be used in conjunction with a solar cell to produce hydrogen,' he explains. The team is now working on modifying the catalyst to reduce the potential at which the electrochemical reaction proceeds and make the system more efficient.
H I Karunadasa, C J Chang and J R Long, Nature, 2010, DOI: 10.1038/nature08969
This abstract is so PC that it hurts.
"whilst"?
If we use sea water to create hydrogen, and make the oceans lower, that should help counteract the melting ice caps from global warming. We will live!
The process is called electrolysis.
in a 100 years, i have no doubt in my mind that the future generation of environmentalist will find fault in the technology thats powering society, even if we adopt everything they support now e.g ethanol, solar panel.
Couldn’t they have just set the oil spill ablaze?
“The process is called electrolysis.”
Yes, and it appears that this catalyst is somewhat more efficient at driving the conversion. This rather begs the question of how much energy is required to convert h20 to hydrogen. One doesn’t get hydrogen for free, and you have to drive the reaction with some form of energy (mostly coal in the US). In the end, I suspect that there is no net energy gain by using hydrogen, as you gotta’ burn the coal to produce the electrons, that drive the reaction.
I’m afraid they’ll have to come up with more than a new catalyst to make hydrogen an economic, or environmental success.
It will cerainly make folks feel good though. ;-)
LOL
We used to use Drano and tin foil - then run the rsulting H2 thru a tube full of soap flakes to dry it and then fill ballons.
Tied shut
with cannon fuse
which we lit off
just before releasing them.
bflr
mark for later.
intelligence looks good on ya
I knew a guy at college who used to do something like that. Only he put the tinfoil and drain cleaner in a plastic 2-liter and sealed it.
Boy howdy, was that thing loud when it went off...
Yes, many technical magazines have lefty editors. The rank and file society members of the respective magazines want the editors ousted. Some examples are: The Journal of the American Institute of Physics, Chemical Engineering News, Nature and Science
So PC in fact he overlooks a very important fact in his first paragraph. The author wrote “ but this method produces the greenhouse gas carbon dioxide.”
Well sonny, there is no more powerful and prevalent greenhouse gas than water vapour, and guess what’s produced when H2 is oxidized to release energy?!?!
Astoundingly weak.
Obtaining hydrogen by electrosis cannot possibly result in any net energy gain. The hydrogen is an energy storage medium, like a rechargable battery, not an energy source and not a particularly efficient one. It does have the advantage of having higher energy density than batteries, though less than gasoline, iirc, measured in joules per kilogram. See: http://en.wikipedia.org/wiki/Hydrogen_economy
The half life of a water molecule in the atmosphere is about a week, a molecule of CO2 about a century. Although molecule for molecule H2O is a more powerful greenhouse gas, it doesn't hang around nearly as long. When was the last time it hailed dry ice?
You know, that statistic about the half life of a given molecule may or may not be correct. But why is it relevant? And do you have a citation for that? (I’m curious)
Also, last time I checked dry ice hail was not a part of the carbon cycle but rain/hail/snow/sleet clearly is a part of the H2O cycle.
I know it’s early, but what’s your point?
As an aside — IF we were to largely switch from carbon based fuels to hydrogen, we’d be pumping huge volumes of water vapor into the atmosphere and probably creating microclimates around major thoroughfares.
BTW, I am all in favor of efficient catalysis to produce hydrogen.
Energy out = energy in - losses
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.