|
Researchers have caught a glimpse of carbonic acid
|
Posted on 11/13/2009 11:09:45 PM PST by neverdem
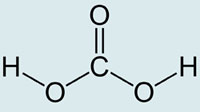
Scientists from Germany and Israel have caught a fleeting glimpse of carbonic acid, the simple yet elusive molecule that plays a key role in nature, from regulating the pH of blood to mediating crucial events in the global carbon cycle. And it appears that the acid is not as weak as the textbooks would have us believe.
Carbonic acid, the hydrated form of carbon dioxide, is an important molecule that is involved in buffering biological fluids such as blood and is a key intermediate in the exchange of carbon dioxide between the atmosphere and the oceans. However, it is so short-lived in solution that its aqueous chemistry has been difficult to study directly.

|
Researchers have caught a glimpse of carbonic acid
|
When CO2 dissolves in water it forms carbonic acid before rapidly dissociating to the bicarbonate anion. The researchers devised a way to generate the acid in a controlled way that would enable it to be observed. They did this by placing bicarbonate ions in the presence of a photoacid - a compound whose acidity can be triggered by a pulse of optical energy. By exciting the photoacid with a shot of laser light for a few tens of femtoseconds, protons are generated which associate with the bicarbonate to form carbonic acid. Femtosecond infrared spectroscopy can then be synchronised with the generation of the acid to get a look at the molecule before it disappears.
By measuring the rate of protonation of the bicarbonate, the researchers were able to gather that the textbook figure for the acidity of carbonic acid is probably significantly inaccurate. 'It is definitely much more acidic than people thought,' says Nibbering. 'When we do the time-resolved experiment we see that its acidity lies somewhere between that of acetic and formic acid.'
Pines says that the study also demonstrates that carbonic acid is more stable than previously thought. This, he says, could have a profound effect on the acidification of the oceans by elevated levels of carbon dioxide in the atmosphere, and could mean the oceans are likely to be significantly more acidic than current models suggest.
Eric Achterberg, a marine biogeochemist at the University of Southampton in the UK, says that the work could have important implications for modelling the sequestration of carbon dioxide under the seabed as part of carbon capture and storage concepts that are being studied. 'An improved knowledge of the acid dissociation constant for carbonic acid will be key in calculations on chemical reactivity of CO2 towards host rocks, and the potential movement of CO2 towards the overlying sea through cracks and faults,' says Achterberg.
Beware carbonic acid!
I'm baffaloed. We don't tell the pH of the oceans by calculating it from a model, we go and test them. They say this sneaky acid hides from the measurements?
We use CO2 to maintain PH at the pool I manage. Works well as long as the calcium hardness is maintained at a 3 to 1 ratio.
How many femtograms of this illegal stuff is considered illegal by the EPA?
>> Beware carbonic acid! <<
Waiting to be safe from Carbonic acid? Don’t hold your breath!
For all you carbonic acid fans, there was a photo of a chuck of solid carbonic acid on the cover of a periodical (either “Science” or “Angewandte Chemie”, I forget which) a number of years back.
Thanks for posting this.
Cheers!
Cheers!
Cheers!
That’s precious - LOL.
Everytime I’ve had to slog my way through CA somebody’s always taken a razor to the swimsuit issue and swiped it - and I’m not yet ready to drop $50,000/yr (or whatever exhorbitant fee they’re now charging) for my own copy!
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.