Skip to comments.
E85 (85% Ethanol) a loser for reduced miles/gallon
The Fargo Forum ^
| 03/04/07
| By Jack F. Carter and John D. Nalewaja
Posted on 03/04/2007 8:01:09 AM PST by Uncle Miltie
E85 is a loser for reduced miles per gallon, as reported in published articles in recent magazines. Stories published in various magazines, e.g., Consumer Reports, CARandDRIVER, Bioscience, Scientific American, American Scientist and Science in 2005 and 2006 question the scientific and economic validity of ethanol (a mixture of gasoline and alcohol) made from corn grain or other fermentable carbohydrates (CHO).
Alcohol made from fermented cellosic material (wood from certain trees, plant materials from plants such as switchgrass or other grasses, etc. may be more feasible. However, cellosic materials are composed of complex CHOs which must be modified to more simple, fermentable CHOs to produce alcohol, and the needed economic procedures are not yet developed.
A significant fact is that gasoline from petroleum has 115,400 British Thermal Units per gallon whereas alcohol (ethanol) has only 75,670 BTUs per gallon, or, alcohol has only .66 the energy of gasoline.
Further, the energy input to produce corn, such as machinery, fertilizer, seed, etc., and the total process of conversion of corn grain to alcohol and by-products requires more energy than is produced in the ethanol, according to researchers at Cornell University (2007 publication) and others. However, others reported a 1.34 gain in energy from the ethanol from the corn when he included the energy of byproducts.
Two publications, Consumer Reports and CARandDRIVER in recent road tests or on an oval track, in 2006 trials found that E85 (gasoline mixed with 85 percent alcohol) has approximately 30 percent less mileage as compared to 87 octane gasoline. At prices of gasoline and E85 in August, 2006, the fuel costs to travel 400 miles (road) with E85 ($3.99) would have exceeded gasoline ($2.49), or a Tahoe Chevrolet went 400 miles on a tankful of gasoline versus the Tahoe going only 290 miles on a tankful of E85.
The author of the story in CARandDRIVER quoted that the Environmental Protection Agency has reported 28 percent reduction in mileage for E85 as compared to gasoline. E85 provided only 0.67 the mileage of gasoline.
Ethanol from corn has required large federal and state subsidies, a 51c/gallon federal subsidy of alcohol blended with gasoline, plus state subsidies and tax incentives to grow to its present 107 ethanol plants producing 5.1 billion gallons of alcohol in 2006, and growing.
The price of corn has increased
50 percent or more in six to nine months benefiting corn growers. The higher price of corn is hurting livestock producers (beef cattle, swine, poultry, etc.) because the price of feeder cattle has decreased significantly and the price of corn for feed has increased 50 percent in six months.
A potentially more efficient producer of liquid fuel energy is thought to be the “cellulosic” system, or production of alcohol from complex CHOs such as wood chips, plant material from corn stalks, and perennial grasses such as switchgrass. However, a basic problem is the development of enzyme(s) to convert complex CHOs to fermentable CHOs.
Economic transportation of such bulky materials also is a problem. Another problem is that the cellulosic plants will use about 500 to 1,000 gallons of water per minute or 1,440,000 gallons per 24 hours with plants closely spaced due to bulk of cellulosic material. (Says Dr. Thomas Robb, in Farm & Ranch Guide, Jan. 5)
The production and use of biodiesel (diesel from petroleum to which are added modified vegetable oils or waste fats) also have economic problems. Canola oil highly publicized for use now has a higher cost per pound or gallon than diesel fuel from petroleum, $3/gallon wholesale versus $2.47/gallon retail. Canola oil is popular for use in cooking or in foods.
Soybean oil has a lower price than canola oil but now has increased to 28.5c/lb. about 10 percent higher than the maximum, 25c/lb. at which using soybean oil in biodiesel will be economic.
The potential users of biofuels are urged to become better informed about their practical and economic feasibility. Stories in the popular press are mostly very favorable to “replaceable, sustainable biofuels” as are corn growers, speculators and most politicians. Other publications are skeptical to negative about the practical and economic feasibility of biofuels now produced from corn grain and other plant sources.
Carter and Nalewaja are professors emeritus in plant science at North Dakota State University.
Both had distinguished careers in teaching and research – Carter in flaxseed for food and fuel, Nalewaja in development of weed control practices. E-mail ImySm@aol.com
TOPICS: Business/Economy; Editorial; US: Minnesota; US: North Dakota
KEYWORDS: energy; ethanol
Navigation: use the links below to view more comments.
first previous 1-20 ... 121-140, 141-160, 161-180 ... 201-203 next last
To: E. Pluribus Unum
Agree. There's a "gold rush" mentality going on in the across the Midwest. Farmers in the corn belt are cash rich for sure. they're buying new pickup's and farm equipment as fast as they can get their hands on them. John Deere must think it's died and gone to heaven. New tractors and combines for everyone! whaaa whooo!!!!!!
To: Mr. Lucky
Ethanol will get worse mileage than gasoline in an engine which is optomized for gasolineethanol will always get worse mileage because it doesn't have as much energy per gallon genius
142
posted on
03/05/2007 5:58:34 PM PST
by
from occupied ga
(Your most dangerous enemy is your own government)
To: from occupied ga
"DO you have anything other than your own opinion to support this?" Read the Argonne National Labs report at the link I posted in #71.
To: A Strict Constructionist
It's become a financial support for Congressmen issue. Follow the money.
A-D-M
144
posted on
03/06/2007 5:26:49 AM PST
by
Kozak
(Anti Shahada: " There is no God named Allah, and Muhammed is his False Prophet")
To: thackney
"Your report, which I've read times you posted before and re-read again, has no support for the numbers it claims. Just some numbers posted on the Internet without the supporting documentation supporting the claim. It has no validity." The link was to a SUMMARY of a longer report by Argonne labs. Follow that up if you want the information. It's peer-reviewed and published information.
Heres another link http://rael.berkeley.edu/EBAMM/FarrellEthanolScience012706.pdf
from the journal "Science", which says the same thing. This is a "scientifically valid" as it gets.
"I've worked in refineries and I understand how much energy it takes. Nothing near this claim."
What did you do?? Fit pipes?? Because you really have no clue as to the processes involved or the energy they take.
"Do you know that gasoline was originally a waste product? Producing Kerosene resulting in gasoline there originally was no market for."
Yes.
"It doesn't take a huge additional amount of energy.
Wrong.
To: LeGrande
"I will try and make it simple and easy for you to understand. Your article included the delivered BTU of the gasoline in the BTU cost of producing the gasoline, because all the BTU's are from a fossil source. It did not include the BTU's from the delivered Ethanol because Ethanol is not a Fossil fuel. If the article was honest it would indicate that it takes 1.73MM BTU's to produce 1 BTU of Ethanol and 1.23MM BTU's to produce 1MM BTU of Gasoline." No. YOU are the one confused. Simply put, in the case of ethanol, you have 1.73MM BTU of "potentially available energy" (some from fossil, some from solar). In the case of gasoline, you have 2.23MM BTU of "potentially available energy" (all from fossil). After both "refining processes" have been done, you end up with 1MM BTU of ethanol and gasoline, having expended 0.73MM BTU to produce the fuel-grade ethanol, and 1.23MM BTU to produce the gasoline.
The link you posted is to a study by a GRADUATE STUDENT, for cripes sake.
See post #145 for a REAL scientific study (which agrees with the Argonne study, BTW).
To: Toddsterpatriot
"Your link? Maybe you'd learn how to make your links active?" What, you're too lazy to cut and paste???
When I'm not pressed for time, I will take the extra trouble to make links active. Lately, I've been pretty busy.
To: Wonder Warthog
So you claim we consume 14,260 Trillion BTU's in fossil fuel to produce the gasoline we refine. And you attribute most of this consumption in the final stage of refinery process after the simple distillation. That is Quadrupole the entire electrical energy the entire industrial market in the US consumes. Where do you claim this power is coming from? This is the big question you are ignoring and proves how foolish your claim is. Such power as you claim is does not exist.
"I've worked in refineries and I understand how much energy it takes. Nothing near this claim."
What did you do?? Fit pipes??
No, I was the lead electrical and instrumentation engineer. I had to provide the main power switchgear, cables and transformers for all the incoming power. I also reviewed all the process flow and instrumentation diagrams for my approval.
"It doesn't take a huge additional amount of energy.
Wrong.
Again and again, where is this power you claim is needed? It doesn't exist because it is not used.
148
posted on
03/06/2007 6:20:07 AM PST
by
thackney
(life is fragile, handle with prayer)
To: Wonder Warthog
In the case of gasoline, you have 2.23MM BTU of "potentially available energy" (all from fossil). You should reread your own links.
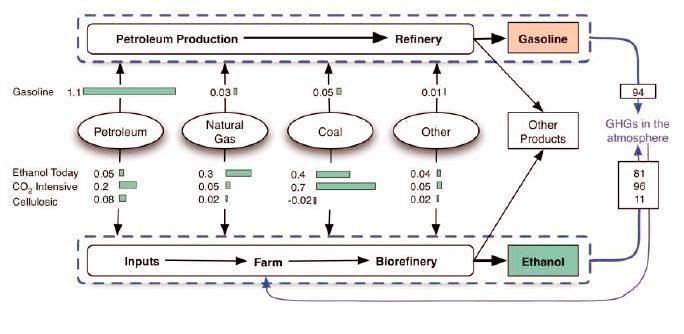
Not only does it count the fuel itself, it also ignores the BTU content of all the other products. And there is a lot of energy in the other products. We do not consume that much energy only for gasoline, we consume that much for gasoline and diesel and jet fuel and kerosene and Petrochem Feedstock and on and on.
149
posted on
03/06/2007 6:32:05 AM PST
by
thackney
(life is fragile, handle with prayer)
To: from occupied ga
Pure ethanol will run in an engine with a compression ration of 19.5:1 If you tried 87 octane gasoline in the same engine, it would barely run, if at all. (the knocking is the sound of the gasoline prematurely igniting, which not only will ruin the engine in short order, but will give horrendous gas mileage until the engine blows)
Genius.
To: Kozak
ADM
I assume that means its always the dam money.
To: A Strict Constructionist
Or in this case Archer Daniels Midland Corp.
152
posted on
03/06/2007 7:16:49 AM PST
by
Kozak
(Anti Shahada: " There is no God named Allah, and Muhammed is his False Prophet")
To: Wonder Warthog
In the case of gasoline, you have 2.23MM BTU of "potentially available energy" (all from fossil).Math is hard.
153
posted on
03/06/2007 7:41:05 AM PST
by
Toddsterpatriot
(Why are protectionists (and goldbugs) so bad at math?)
To: Wonder Warthog
I went to your site. The Argonne study. Figure 2, if you add up all the numbers indicates that it takes 1.19 energy units to produce 1 energy unit of gasoline and 1.98 energy units to produce 1 energy unit of Ethanol. It takes more coal energy alone to produce the enthanol energy than you get out of it. LOL
Now lets look at your funny numbers.
After both "refining processes" have been done, you end up with 1MM BTU of ethanol and gasoline, having expended 0.73MM BTU to produce the fuel-grade ethanol, and 1.23MM BTU to produce the gasoline.
Let's pretend for a second that you are right and let us convert the units to gallons to make it easier to understand :) You are stating that it take 1.23 gallons of gasoline to manufacture one gallon of gasoline. So for every gallon sold 1.23 gallons was used to get it to the cars gas tank.
A barrel of crude (let us assume that it all becomes gas) costs 60 dollars and contains 42 gallons that is $1.43 per gallon. If we use your numbers then it takes 1.23 gallons to make the gas and 1 gallon for the gas. 1.23+1=2.23 gallons. At $1.43 per gallon the cost to produce a gallon of gasoline is $1.43 x 2.23 = $3.19. That is for the cost of the raw material alone. Do you really believe that Oil companies sell gas at a loss?
Even with your lack of reading comprehension, I don't think you are that stupid. Take a step back and think about what you are saying. Does it make sense to you that out of a barrel of oil, 70% of it is consumed to make 30%?
154
posted on
03/06/2007 7:49:11 AM PST
by
LeGrande
(Muslims, Jews and Christians all believe in the same God of Abraham.)
To: Kozak
I was just making fun of ADM but being serious about politicians. I live a mile from the Mississippi and you can't miss their facilities.
To: Mr. Lucky
Pure ethanol will run in an engine with a compression ration of 19.5:1 If you tried 87 octane gasoline in the same engine, it would barely run, if ...So what? None of this is relevant. If you'd ever heard of the first law of thermodynamics you'd know know too. It takes energy to accelerate mass and overcome friction. Engines are limited in their efficiency by the theoretical limit of their cycle. Changing the compression ration helps a little bit, but the basic limitations are available energy in the fuel and mass of the vehicle. Ie going from 10 or 11 to 1 to 19 to one doesn't make a lot of difference because of the law of diminishing returns. Here
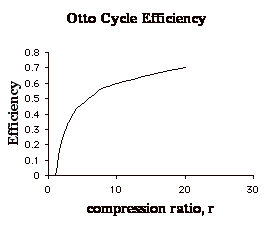
is how compression affects efficiency for an otto cycle. You can read the whole thing at http://ocw.mit.edu/ans7870/16/16.unified/thermoF03/chapter_5.htm Increase mass = worse MPG, decrease fuel energy = worse MPG. Increase compression over the 10 - 11 range in most modern vehicles = SLIGHTLY increased MPG
156
posted on
03/06/2007 8:34:03 AM PST
by
from occupied ga
(Your most dangerous enemy is your own government)
To: from occupied ga
That's all fine, but at 19.5:1 compression ration, the engine will operate more efficiently on ethanol than 87 octane gasoline.
To: Mr. Lucky
but at 19.5:1 compression ration, the engine will operate more efficiently on ethanol than 87 octane gasoline.As you pointed out you can't operate at 19.5 to one on 87 octane, so yes, but you'll still get better gas mileage at 10:1 on 87 octane than at 19.5:1 on etoh
158
posted on
03/06/2007 8:59:16 AM PST
by
from occupied ga
(Your most dangerous enemy is your own government)
To: from occupied ga
I should have mentioned that you can Google on something to the effect of "High efficiency Engine Technologies for Alcohol Fuel" to review the EPA's actual results from increasing compression ratios.
To: Wonder Warthog
you have 2.23MM BTU of "potentially available energy" (all from fossil). At their press release:
Argonne expert addresses energy, environmental impacts of fuel ethanol
There is a short presentation that walks through the comparison of energies required for different fuels. It also compares fuels used for electrical power generation. This makes it clear the 1.23 MMBTU to produce 1 MMBTU includes the BTU of the fuel itself.
160
posted on
03/06/2007 11:35:35 AM PST
by
thackney
(life is fragile, handle with prayer)
Navigation: use the links below to view more comments.
first previous 1-20 ... 121-140, 141-160, 161-180 ... 201-203 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson

