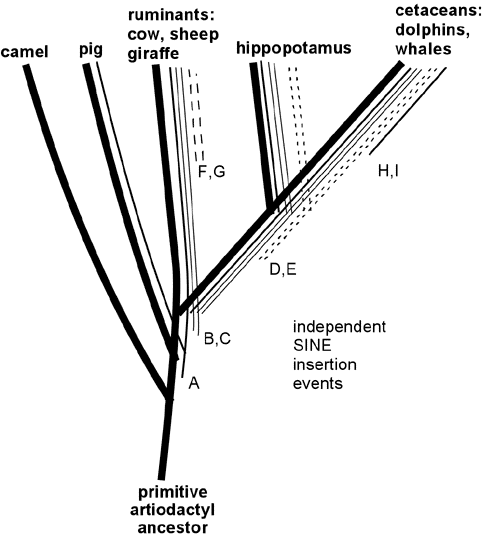
Plus, "nearly all scientists" (including at least 99% of biologists) conclude (from the evidence) that evolution can and does explain the variations across species. You forgot to mention that part.
There is very little evidence that natural selection can explain how a fish could turn into a cow or even a human.
Why are you posting falsehoods? You're obvously massively ignorant of the actual evidence, because otherwise you couldn't possibly say such a thing with a straight face. So clearly you're just parroting someone else's lie. Whose is it? Where did you learn this non-fact?
The intermediate species just don't exist...even considering the limited fossil record we have. There should be several intermediate examples out there. All we have is archeoptryx...not much IMHO.
...and here we see a specific example of your gross ignorance. Oh, so Archaeopteryx is "all" we have in the way of intermediate fossil species, eh? I see...
Here, educate yourself: Transitional Vertebrate Fossils FAQ.
Fossil Hominids: The Evidence for Human Evolution.
Where exactly is the "missing" transition in the following sequence? It looks pretty complete and gradual to me -- certainly there's no sudden "jump", no discontinuity, no pair between which a creationist would have any trouble dismissing such a small amount of change as "just microevolution", "just variation within a kind":
(The above is from 29 Evidences for Macroevolution -- Part 1: The Unique Universal Phylogenetic Tree)Figure 1.4.4. Fossil hominid skulls. Some of the figures have been modified for ease of comparison (only left-right mirroring or removal of a jawbone). (Images © 2000 Smithsonian Institution.)
- (A) Pan troglodytes, chimpanzee, modern
- (B) Australopithecus africanus, STS 5, 2.6 My
- (C) Australopithecus africanus, STS 71, 2.5 My
- (D) Homo habilis, KNM-ER 1813, 1.9 My
- (E) Homo habilis, OH24, 1.8 My
- (F) Homo rudolfensis, KNM-ER 1470, 1.8 My
- (G) Homo erectus, Dmanisi cranium D2700, 1.75 My
- (H) Homo ergaster (early H. erectus), KNM-ER 3733, 1.75 My
- (I) Homo heidelbergensis, "Rhodesia man," 300,000 - 125,000 y
- (J) Homo sapiens neanderthalensis, La Ferrassie 1, 70,000 y
- (K) Homo sapiens neanderthalensis, La Chappelle-aux-Saints, 60,000 y
- (L) Homo sapiens neanderthalensis, Le Moustier, 45,000 y
- (M) Homo sapiens sapiens, Cro-Magnon I, 30,000 y
- (N) Homo sapiens sapiens, modern
Evolutionary theory predicted that there should be transitional forms between the reptilian-style jaw joint and the mammalian-style jaw joint (the earliest mammals evolved from reptiles). For years creationists crowed about the "missing links", and made their own predictions that not only would there be no such transitional fossils found, but that any creature with a jaw that was transitional between that of reptiles and mammals would die of starvation, since such a "half and half" jaw joint was "obviously" mechanically unworkable. Nonetheless, biologists kept searching for the fossils predicted by evolution, and not only found one or two, but found a *wealth* of them which provide a *very* complete and smooth transitional sequence -- exactly as evolution predicted. Oddly enough, I never hear the creationists bring that one up...
Evolution predicted that transitional forms once existed between dinosaurian forelimbs and bird wings. Creationists predicted that "half a wing" would be unworkable and useless. Guess whose predictions were found to be right?Reptile -> Mammal evolutionary transition:
The above is from 29+ Evidences for Macroevolution, which compiles several hundred transitional fossils, which is itself just a *SMALL* sampling of the ENORMOUS numbers of fine transitional sequences found in the fossil record and well known to anyone who has bothered to CRACK OPEN A BOOK -- or even do a websearch -- in the past 25 years or so... So what's the anti-evolutionists' excuse for remaining abysmally ignorant of such things, and repeatedly making the false claim that there are "no" transitional fossils, etc.?Example 2: reptile-mammals
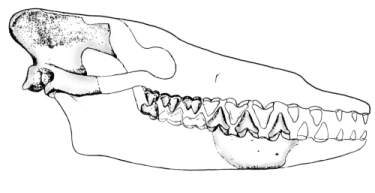
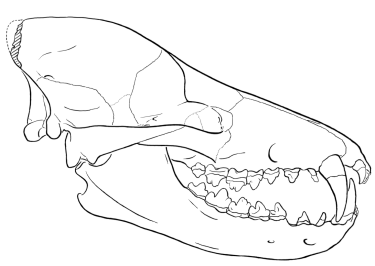
We also have an exquisitely complete series of fossils for the reptile-mammal intermediates, ranging from the pelycosauria, therapsida, cynodonta, up to primitive mammalia (Carroll 1988, pp. 392-396; Futuyma 1998, pp. 146-151; Gould 1990; Kardong 2002, pp. 255-275). As mentioned above, the standard phylogenetic tree indicates that mammals gradually evolved from a reptile-like ancestor, and that transitional species must have existed which were morphologically intermediate between reptiles and mammals—even though none are found living today. However, there are significant morphological differences between modern reptiles and modern mammals. Bones, of course, are what fossilize most readily, and that is where we look for transitional species from the past. Osteologically, two major striking differences exist between reptiles and mammals: (1) reptiles have at least four bones in the lower jaw (e.g. the dentary, articular, angular, surangular, and coronoid), while mammals have only one (the dentary), and (2) reptiles have only one middle ear bone (the stapes), while mammals have three (the hammer, anvil, and stapes) (see Figure 1.4.1).
Early in the 20th century, developmental biologists discovered something that further complicates the picture. In the reptilian fetus, two developing bones from the head eventually form two bones in the reptilian lower jaw, the quadrate and the articular (see the Pelycosaur in Figure 1.4.1). Surprisingly, the corresponding developing bones in the mammalian fetus eventually form the anvil and hammer of the unique mammalian middle ear (also known more formally as the incus and malleus, respectively; see Figure 1.4.2) (Gilbert 1997, pp. 894-896). These facts strongly indicated that the hammer and anvil had evolved from these reptilian jawbones—that is, if common descent was in fact true. This result was so striking, and the required intermediates so outlandish, that many anatomists had extreme trouble imagining how transitional forms bridging these morphologies could have existed while retaining function. Young-earth creationist Duane Gish stated the problem this way:
"All mammals, living or fossil, have a single bone, the dentary, on each side of the lower jaw, and all mammals, living or fossil, have three auditory ossicles or ear bones, the malleus, incus and stapes. ... Every reptile, living or fossil, however, has at least four bones in the lower jaw and only one auditory ossicle, the stapes. ... There are no transitional fossil forms showing, for instance, three or two jawbones, or two ear bones. No one has explained yet, for that matter, how the transitional form would have managed to chew while his jaw was being unhinged and rearticulated, or how he would hear while dragging two of his jaw bones up into his ear." (Gish 1978, p. 80)
Gish was incorrect in stating that there were no transitional fossil forms, and he has been corrected on this gaff numerous times since he wrote these words. However, Gish's statements nicely delineate the morphological conundrum at hand. Let's review the required evolutionary conclusion. During their evolution, two mammalian middle ear bones (the hammer and anvil, aka malleus and incus) were derived from two reptilian jawbones. Thus there was a major evolutionary transition in which several reptilian jawbones (the quadrate, articular, and angular) were extensively reduced and modified gradually to form the modern mammalian middle ear. At the same time, the dentary bone, a part of the reptilian jaw, was expanded to form the major mammalian lower jawbone. During the course of this change, the bones that form the hinge joint of the jaw changed identity. Importantly, the reptilian jaw joint is formed at the intersection of the quadrate and articular whereas the mammalian jaw joint is formed at the intersection of the squamosal and dentary (see Figure 1.4.1).
How could hearing and jaw articulation be preserved during this transition? As clearly shown from the many transitional fossils that have been found (see Figure 1.4.3), the bones that transfer sound in the reptilian and mammalian ear were in contact with each other throughout the evolution of this transition. In reptiles, the stapes contacts the quadrate, which in turn contacts the articular. In mammals, the stapes contacts the incus, which in turn contacts the malleus (see Figure 1.4.2). Since the quadrate evolved into the incus, and the articular evolved into the malleus, these three bones were in constant contact during this impressive evolutionary change. Furthermore, a functional jaw joint was maintained by redundancy—several of the intermediate fossils have both a reptilian jaw joint (from the quadrate and articular) and a mammalian jaw joint (from the dentary and squamosal). Several late cynodonts and Morganucodon clearly have a double-jointed jaw. In this way, the reptilian-style jaw joint was freed to evolve a new specialized function in the middle ear. It is worthy of note that some modern species of snakes have a double-jointed jaw involving different bones, so such a mechanical arrangement is certainly possible and functional.
Since Figure 1.4.3 was made, several important intermediate fossils have been discovered that fit between Morganucodon and the earliest mammals. These new discoveries include a complete skull of Hadrocodium wui (Luo et al. 2001) and cranial and jaw material from Repenomamus and Gobiconodon (Wang et al. 2001). These new fossil finds clarify exactly when and how the malleus, incus, and angular completely detached from the lower jaw and became solely auditory ear ossicles.
Recall that Gish stated: "There are no transitional fossil forms showing, for instance, three or two jawbones, or two ear bones" (Gish 1978, p. 80). Gish simply does not understand how gradual transitions happen (something he should understand, obviously, if he intends to criticize evolutionary theory). These fossil intermediates illustrate why Gish's statement is a gross mischaracterization of how a transitional form should look. In several of the known intermediates, the bones have overlapping functions, and one bone can be called both an ear bone and a jaw bone; these bones serve two functions. Thus, there is no reason to expect transitional forms with intermediate numbers of jaw bones or ear bones. For example, in Morganucodon, the quadrate (anvil) and the articular (hammer) serve as mammalian-style ear bones and reptilian jaw bones simultaneously. In fact, even in modern reptiles the quadrate and articular serve to transmit sound to the stapes and the inner ear (see Figure 1.4.2). The relevant transition, then, is a process where the ear bones, initially located in the lower jaw, become specialized in function by eventually detaching from the lower jaw and moving closer to the inner ear.
Here's another look:
(The above is from The Fossil Record: Evolution or "Scientific Creation", which is yet ANOTHER source the anti-evolutionists are obviously completely ignorant of -- not that that stops them from spouting off falsehoods about the subject anyway...Mammal-Like Reptiles
As previously stated, a succession of transitional fossils exists that link reptiles (Class Reptilia) and mammals (Class Mammalia). These particular reptiles are classifie as Subclass Synapsida. Presently, this is the best example of th e transformation of one major higher taxon into another. The morphologic changes that took place are well documented by fossils, beginning with animals essentially 100% reptilian and resulting in animals essentially 100% mammalian. Therefore, I have chosen this as the example to summarize in more detail (Table 1, Fig. 1).
![[Fig. 1b]](http://www.gcssepm.org/images/fossil_b.gif)
Skulls and jaws of synapsid reptiles and mammals; left column side view of skull; center column top view of skull; right column side view of lower jaw. Hylonomus modified from Carroll (1964, Figs. 2,6; 1968, Figs. 10-2, 10-5; note that Hylonomus is a protorothyrod, not a synapsid). Archaeothyris modified from Reisz (1972, Fig. 2). Haptodus modified from Currie (1977, Figs, 1a, 1b; 1979, Figs. 5a, 5b). Sphenacodo n modified from Romer & Price (1940, Fig. 4f), Allin (1975, p. 3, Fig. 16);note: Dimetrodon substituted for top view; modified from Romer & Price, 1940, pl. 10. Biarmosuchus modified from Ivakhnenko et al. (1997, pl. 65, Figs. 1a, 1B, 2); Alin & Hopson (1992; Fig. 28.4c); Sigogneau & Tchudinov (1972, Figs. 1, 15). Eoarctops modified from Broom (1932, Fig. 35a); Boonstra (1969, Fig. 18). Pristerognathus modified from Broom (1932, Figs 17a, b,c); Boonstra (1963, Fig. 5d). Procynosuchus modified from Allin & Hopson (1992, Fig. 28.4e); Hopson (1987, Fig. 5c); Brink (1963, Fig. 10a); Kemp (1979, Fig. 1); Allin (1975, p. 3, Fig. 14). Thrinaxodon modified from Allin & Hopson (1992, Fig. 28.4f);Parrington (1946, Fig. 1); Allin (1975, p. 3, Fig. 13). Probainognathus modified from Allin & Hopson (1992, Fig. 28.4g); Romer (1970, Fig. 1); Allin (1975, p. 3, Fig. 12). Morga nucodon modified from Kermack, Mussett, & Rigney (1981, Figs. 95, 99a; 1973, Fig. 7a); Allin (1975, p. 3, Fig. 11). Asioryctes modified from Carroll (1988, Fig. 20-3b). Abbreviations: ag = angular; ar = articular; cp = coronoid process; d = dentary; f = lateral temporal fenestra; j = jugal; mm = attachment site for mammalian jaw muscles; o = eye socket; po = post orbital; q = quadrate; rl = reflected lamina; sq = squamosal; ty = tympanic.
TAXONOMY LATERAL TEMPORAL FENESTRA LOWER JAW DENTARY TEETH LOWER JAW: POST- DENTARY BONES MIDDLE EAR & JAW ARTICULATION M: Early Placental mammals
Asioryctes
Upper CretaceousMerged with eye socket; cheek arch bowed out laterally 100% of jaw length is the den- tary; condylar process in contact with squamosal Fully differentiated teeth; incisors, canines, premolars; one tooth replacement No post-dentary bones 3 middle ear bones (stapes, incus, malleus) + tympanic; squamosal-dentary jaw joint L: "Pantothere" mammals
Amphitherium
Middle/Upper JurassicX 100% of jaw length is the den- tary; condylar process contacts squamosal Fully differentiated teeth; incisors, canines, premolars; one tooth replacement Post-dentary bones migrated to middle ear Probably 3 middle ear bones (stapes, incus, malleus) + tympanic; squamosal-dentary jaw joint K: Morganucodontid mammals
Morganucodon Upper Triassic & Lower JurassicMerged with eye socket; cheeck arch bowed out laterally 100% of jaw length is the den- tary; condylar process expanded posteriorly to make contact with squamosal Fully differentiated teeth; incisors, canines, premolars; one tooth replacement 20% of jaw length; reflected lamina decreased to narrow ribbon-like horseshoe Stapes extends from inner ear capsule to quadrate; quadrate tiny; both quadrate-articular and squamosal-dentary jaw joints J: Chiniquodontid cynodonts
Probainognathus
Middle TriassicMuch larger than eye socket; 40- 45% of skull length; expanded posterioirly, medially, & laterally; midline of skull narrow sagittal crest; chek arch bowed out laterally 95% of jaw length is the dentary; large coronoid process expanded posteriorly; condylar process expanded posteriorly Large single canine; cheek teeth multicusped; tooth replacement reduced 20% of jaw length; angular notch widened ventrally; width of main part of angular decreased; reflec - ted lamina decreased to narrow ribbon-like horseshoe Stapes extends from inner ear capsule to quadrate; quadrate tiny; quadrate-articular joint I:Galesaurid cynodonts
Thrinaxodon
Lower TriassicMuch larger than eye socket; 40% of skull length; expanded pos- terioirly, medially, & laterally; midline of skull narrow sagittal crest; chek arch bowed out laterally 85% of jaw length is the dentary; large coronoid process expanded to top of eye socket and pos- teriorly; jaw muscles attached to most of coronoid process Large single canine; cheek teeth multicusped; tooth replacement reduced 25% of jaw length; angular notch widened ventrally; width of reflec- ted lamina decreased; width of main part of angular decreased Stapes extends from inner ear capsule to quadrate; quadrate small; quadrate-articular jaw joint H: Procynosuchid cynodonts
Procynosuchus
upper Upper PermianMuch larger than eye socket; 40% of skull length; expanded pos- terioirly, medially, & laterally; midline of skull narrow sagittal crest; chek arch bowed out laterally 75-80% of jaw length is the den- tary; coronoid process expanded to near top of eye socket and posteriorly; jaw muscles attached to dorsal part of coronoid process Large single canine; cheek teeth multicusped 30% of jaw length; angular notch widened ventrally; width of reflected lamina decreased Stapes extends from inner ear capsule to quadrate; quadrate small; quadrate-articular jaw joint G: Early Therocephalians
Pristerognathus
lower Upper PermianLarger than eye socket; expanded posteriorly and medially; 30% of skull length 75-80% of jaw length is the den- tary; posterior end of dentary expanded posteriorly and dorsally into narrow blade-like coronoid process; rises to middle of eye socket Large single canine; other teeth simple cones. 35% of jaw length; angular notch deepened into a cleft; reflected lamina large, broad, blade-like Stapes extends from inner ear capsule to quadrate; quadrate small; quadrate-articular jaw joint F: Early Gorgonopsians
Eoarctops
lower Upper PermianSlightly larger than eye socket; expanded posteriorly and medially (minimal); 20-25% of skull length 65-75% of jaw length is the den- tary; posterior end of dentary slightly expanded posteriorly and dorsally as incipient coronoid process Large single canine; other teeth simple cones. 40% of jaw length; angular notch deepened into a cleft; reflected lamina large, broad, blade-like Stapes extends from inner ear capsule to quadrate; quadrate- articular jaw joint E: Eotitanosuchians
Sphenacodon
Lower PermianSmall; slightly smaller than eye socket; slightly expanded posteriorly and medially 65-75% of jaw length is the den- tary; posterodorsal edge rises broadly but slightly above tooth row Large single canine; other teeth simple cones. 40% of jaw length; angular notch deepened into a cleft; reflected lamina large, broad, blade-like Stapes extends from inner ear capsule to quadrate; quadrate- articular jaw joint D: Late sphenacodonts
Sphenacodon
Upper PennsylvanianSmall; smaller than eye socket; confined to one side of skull 65% of jaw length is the dentary; posterodorsal edge rises broadly but slightly above the tooth row Enlarged incipient canines; other teeth simple cones 60% of jaw length; venntral edge of angular notched ("angular notch") offsetting a short pro- tusion (reflected lamina) Stapes extends from inner ear capsule to quadrate; quadrate large and plate-like; quadrate- articular jaw joint C: Early spenacodonts
Haptodus
Upper PennsylvanianTiny; smaller than eye socket; confined to one side of skull 65-75% of jaw length is the den- tary; posterodorsal edge rises broadly but slightly above tooth row Undifferentiated; slightly enlarged incipient canines just behind nares 70% of jaw length; ventral edge of angular with shallow indentation Stapes extends from inner ear capsule to quadrate; quadrate- articular jaw joint B: Early ophiacodonts
Archaothyris
upper Middle PennsylvanianTiny; smaller than eye socket; confined to one side of skull x Undifferentiated; slightly enlarged incipient canines just behind nares x Stapes extends from inner ear capsule to quadrate; quadrate- articular jaw joint A: Protorothyrids
Hylonomus
lower Middle PennsylvanianAbsent 65-75% of jaw length is the den- tary; posterodorsal edge rises broadly but slightly above tooth row Undifferentiated; slightly enlarged incipient canines just behind nares 70% of jaw length; ventral edge of angular continuous Stapes extends from inner ear capsule to quadrate; quadrate- articular jaw joint
Table 1: Morphology of synapsid reptiles and mammals (Note that Hylonomus is a protothyrid, not a synapsid). Data from references cited in text.
Modern reptiles and mammals are very distinctive, easily diagnosable, and do not intergrade. Reptiles are covered by scales, mammals by hair; reptiles are cold-blooded, mammals warm-blooded; reptiles do not suckle their young, mammals have mammary glands; reptiles have sprawling posture, mammals have upright posture. Most of these features are soft part anatomy or physiology that very rarely fossilize (although dinosaur skin impressions are known from Cretaceous sediments, and imprints of mammal hair are known from Eocene bats from Germany; Franzen, 1990). In the fossil record, we must look to skeletal features.
There are many skeletal features which allow us to distinguish the reptiles from the mammals (Carroll, 1988; Table 1, rows A, M). The single most important defining characteristic is the nature of the articulation of the lower jaw to the skull (Simpson, 1959). In reptiles, multiple bones comprise the lower jaw. A small bone at the posterior end of the lower jaw, the articular, articulates with the quadrate bone of the skull (Simpson, 1959; Carroll, 1988). In mammals, one large bone, the dentary, comprises the lower jaw. It articulates with the squamosal bone of the skull (Simpson, 1959; Carroll, 1988).
From comparative anatomy studies, it is certain that most of the bones of the reptiles and mammals are homologous (Crompton & Parker, 1978; Carroll, 1988). Of greatest importance, the middle ear bones of mammals (stapes, incus, malleus, and tympanic) are homologous with several of the skull and jaw bones of reptiles (stapes, quadrate, articular, and angular, respectively; Romer, 1956, p. 33-38, 1970a; Allin, 1975, 1986; Allin & Hopson, 1992; Crompton & Parker, 1978; Hopso n, 1987, 1994; Carroll, 1988). One group of reptiles, the synapsids (Subclass Synapsida), share with the mammals an additional homologous structure: the lateral temporal fenestra, which is an opening in the skull behind the eye socket at the triple junction between the squamosal, jugal , and post orbital bones (Broom, 1932; Frazetta, 1968; Kemp, 1982; Carroll, 1988). A band of bone composed of the jugal and the squamosal is adjacent to the lateral temporal fenestra (Broom, 1932; Kemp, 1982; Carroll, 1988). This is the cheek arch so characteristic of mammal skulls (Broom, 1932; Kemp, 1982; Carroll, 1988). Therefore, synapsids are commonly named the “mammal-like reptiles.”
The presence of diagnosable morphologic differences between reptiles (including the oldest reptiles and the oldest synapsids) and mammals distinguishes them as distinct taxa. This allows us to test evolution by looking for transitional forms between the two. Because many of the bones are homologous, we should find evidence illustrating how these bones were modified over time to become the new bones. Furthermore, these morphologic changes should happen in parallel and in geochronologic succession.
Synapsid reptiles inhabited Pangea from the Middle Pennsylvanian through the Early Jurassic (Kemp, 1982, 1985; Sloan, 1983; Carroll, 1988; Hopson, 1969, 1987, 1994; Hopson & Crompton, 1969; Hotton, et al., 1986; Crompton & Jenkins, 1973; Sidor & Hopson, 1998; Romer & Price, 1940; Broom, 1932; Boonstra, 1963, 1969, 1971; Tchudinov, 1983; Olson, 1944; Tatarinov, 1974; Vyushkov, 1955; Efremov, 1954). From the Early Permian through the Early Triassic, they were the largest and most abundant land animals (Sloan, 1983; Colbert, 1965). Though much less well known to the general public than dinosaurs, one of the “cereal box dinosaurs,” Dimetrodon (the sail-backed reptile), is a synapsid, not a dinosaur (Romer & Price, 1940; Carroll, 1988). The oldest mammals are Late Triassic (Kemp, 1982; Carroll, 1988). Below is a discussion of the geochronologic succession linking synapsids and mammals. The oldest reptiles (named protorothyrids; Carroll, 1964, 1988, p. 192-199) are from the lower Middle Pennsylvanian, and the oldest synapsids (Reisz, 1972) are from the upper Middle Pennsylvanian, both of Nova Scotia. Upper Pennsylvanian and Lower Permian forms are known primarily from the midcontinent and Permian Basin region of the United States (Romer & Price, 1940; Currie, 1977, 1979; Kemp, 1982; Sloan, 1983). The basal Upper Permian forms are known from Russia (Tchudinov, 1960, 1983; Efremov, 1954; Olson, 1962; Sigogneau & Tchudinov, 1972; Ivakhnenko et al., 1997). Most of the Upper Permian and Lower Triassic succession is known from southern Africa, especially the Great Karoo of South Africa (Broom, 1932; Boonstra, 1963, 1969, 1971; Hopson & Kitching, 1972; Kemp, 1982; Sloan, 1983). The Middle Triassic forms are from South America (Romer, 1969a, 1969b, 1970b, 1973; Romer & Lewis, 1973; Bonaparte & Barbarena, 1975), and the Upper Triassic and Lower Jurassic mammals are known from Eurasia (Kermack, Mussett, & Rigney, 1973, 1981; Kemp, 1982). Subsequent Mesozoic mammals are known from all over the world (Simpson, 1928; Lillegraven et al., 1979).
When placed in proper geochronologic succession, the synapsids naturally form a succession of taxa (genera and families) that progressively become more mammal-like and less reptile-like (Kemp, 1982, 1985; Sloan, 1983; Sidor & Hopson, 1998; Hopson, 1987, 1994). Morphologic changes, summarized in Table 1 and Figure 1, affect the entire skeletal anatomy of these animals, but are most clearly displayed in their skulls.
The lateral temporal fenestra increased in size from a tiny opening smaller than the eye socket to a giant opening occupying nearly half the length of the skull. Ultimately, it merged with the eye socket, thus producing the full development of the cheek arch so characteristic of mammals (Broom, 1932; Frazetta, 1968; Kemp, 1982; Sloan, 1983; Hopson, 1987, 1994; Carroll, 1988).
Successively, the relative proportion of the lower jaw comprised of the dentary bone (teeth-bearing bone) gradually increased until the entire lower jaw consisted of the dentary (Kemp, 1982; Sloan, 1983; Carroll, 1988; Hopson, 1987, 1994). In Pennsylvanian and Lower and basal Upper Permian synapsids, the postero-dorsal edge of the lower jaw rose broadly but only slightly above the level of the tooth row (Romer & Price, 1940; Currie, 1977, 1979; Ivakhnenko et al., 1997; Tchudinov, 1960, 1983; Efremov, 1954; Olson, 1962; Sigogneau & Tchudinov, 1972; Hopson, 1987, 1994). In succeeding forms, the posterior part of the dentary expanded dorsally and posteriorly as a blade-like process, and progressively became larger (Broom, 1932; Boonstra, 1963, 1969, 1971; Sigogneau, 1970; Brink, 1963; Kemp, 1979; Hopson, 1987, 1994), forming the coronoid process (Parrington, 1946; Fourie, 1974; Romer, 1969b, 1970b, 1973; Hopson, 1987, 1994) to which the mammalian-type jaw musculature is attached (Barghusen, 1968; Bramble, 1978; Crompton, 1972; Crompton & Parker, 1978; Kemp, 1982; Sloan, 1983; Carroll, 1988). Concomitantly, the post-dentary bones progressively reduced in size (Allin, 1975; Crompton, 1972; Crompton & Parker, 1978; Kemp, 1982; Sloan, 1983; Carroll, 1988; Hopson, 1987, 1994).
Beginning with the Upper Pennsylvanian sphenacodonts, a notch developed in the angular bone that offsets a projection, the reflected lamina (Allin, 1975; Allin & Hopson, 1992; Hopson, 1987, 1994; Romer & Price, 1940; Currie, 1977, 1979; Kemp, 1982; Sloan, 1983; Carroll, 1988). The reflected lamina first became a large blade-like flange (Allin, 1975; Allin & Hopson, 1992; Hopson, 1987, 1994; Ivakhnenko et al., 1997; Tchudinov, 1960, 1983; Efremov, 1954; Olson, 1962; Sigogneau & Tchudinov, 1972; Broom, 1932; Sigogneau, 1970; Boonstra, 1963, 1969, 1971), and then was progressively reduced to a delicate horseshoe-shaped bone (Allin, 1975; Allin & Hopson, 1992; Hopson, 1987, 1994; Brink, 1963; Parrington, 1946; Fourie, 1974; Romer, 1969b, 1970b, 1973; Kermack, Mussett, & Rigney, 1973, 1981; Kemp, 1979, 1982; Sloan, 1983; Carroll, 1988).
Simultaneously, the quadrate progressively decreased in size (Allin, 1975; Allin & Hopson, 1992; Hopson, 1987, 1994; Kemp, 1982; Sloan, 1983; Carroll, 1988). The articular did not decrease in size much, being small initially, but developed a downward-pointing prong (Allin, 1975; Allin & Hopson, 1992; Hopson, 1987, 1994; Kemp, 1982; Sloan, 1983; Carroll, 1988). In the synapsids, the lower jaw was hinged to the skull by the articular and quadrate bones (Crompton, 1972; Crompton & Parker, 1978; Allin, 1975; Allin & Hopson, 1992; Hopson, 1987, 1994). Thus they are classified as reptiles (Simpson, 1959; Kemp, 1982; Sloan, 1983; Carroll, 1988). As the quadrate and articular became smaller, they were relieved of their solid suture to the dentary and skull (Crompton, 1972; Allin, 1975, 1986; Allin & Hopson, 1992; Hopson, 1987, 1994; Crompton & Parker, 1978; Kemp, 1982; Sloan, 1983; Carroll, 1988). A projection of the dentary extended posteriorly and made contact with the squamosal. Morganucodon possessed the mammalian dentary-squamosal jaw joint adjacent to the reptilian articular-quadrate jaw joint (Kermack, Mussett, & Rigney, 1973, 1981; Carroll, 1988). It is classified as the first mammal, but it is a perfect intermediate. Now that a new jaw joint was established, the quadrate and articular were subsequently relieved of that function (Crompton, 1972; Allin, 1975, 1986; Allin & Hopson, 1992; Hopson, 1987, 1994; Crompton & Parker, 1978; Kemp, 1982; Sloan, 1983; Carroll, 1988). Ultimately, in Middle and Upper Jurassic mammals, the tiny quadrate, articular, and ring-like angular migrated as a unit to the middle ear where they joined the stapes and became the incus, malleus, and tympanic bones (Allin, 197 5, 1986; Allin & Hopson, 1992; Hopson, 1987, 1994; Kemp, 1982; Sloan, 1983; Carroll, 1988).
Progressively, the teeth became differentiated. The large canines developed first, followed by the development of multicusped cheek teeth, reduced tooth replacement (Osborn & Crompton, 1973; Crompton & Parker, 1978), and finally full y differentiated incisors, canines, premolars, and molars with one tooth replacement during life (Kemp, 1982; Hopson, 1994).
Many other morphologic changes are documented in the fossil record. These demonstrate the morphologic and geochronologic succession from sprawling limb posture to upright limb posture of mammals (Jenkins, 1971; Romer & Lewis, 197 3; Kemp, 1982; Carroll, 1988; Hopson, 1994). As Jenkins (1971, p. 210) stated, “In details of morphology and function, the cynodont post-cranial skeleton should be regarded as neither ‘reptilian’ nor ‘mammalian’ but as transitional between the two classes .” Other changes have been adequately summarized elsewhere (Kemp, 1982; Sloan, 1983; Carroll, 1988; Hopson, 1994). Obviously, fundamental physiologic changes must have taken place as well, many of which are not directly preserved in the fossil record, though some can be inferred from the skeletal anatomy (Findlay, 1968; Kemp, 1982; Sloan, 1983, Carroll, 1988; Hopson, 1994).
This is well documented in the fossil record by a massive volume of incontrovertible data that cannot be explained away. Such large-scale, progressive, continuous, gradual, and geochronologically successive morphologic change (Sidor & Hopson, 1998) is descent with modification, and provides compelling evidence for evolution on a grand scale.
Theropod dinosaur to bird evolutionary transition:
The cladogram for the evolution of flight looks like this:
(Note -- each name along the top is a known transitional fossil; and those aren't all that have been discovered.) Here's a more detailed look at the middle section:
Fossils discovered in the past ten years in China have answered most of the "which came first" questions about the evolution of birds from dinosaurs.
We now know that downy feathers came first, as seen in this fossil of Sinosauropteryx:
That's a close-up of downy plumage along the backbone. Here's a shot of an entire fossil
Sinosauropteryx was reptilian in every way, not counting the feathers. It had short forelimbs, and the feathers were all the same size. Presumably, the downy feathers evolved from scales driven by a need for bodily insulation.
Next came Protarchaeopteryx:
It had long arms, broad "hands", and long claws:
Apparently this species was driven by selection to develop more efficient limbs for grasping prey. One of the interesting things about this species is that the structure of the forelimb has been refined to be quite efficient at sweeping out quickly to grab prey, snap the hands together, then draw them back towards the body (mouth?). The specific structures in question are the semilunate carpal (a wrist bone), that moves with the hand in a broad, flat, 190 degree arc, heavy chest muscles, bones of the arm which link together with the wrist so as to force the grasping hands to spread out toward the prey during the forestroke and fold in on the prey during the upstroke. Not only is this a marvelously efficient prey-grabbing mechanism, but the same mechanism is at the root of the wing flight-stroke of modern birds. Evolution often ends up developing a structure to serve one need, then finds it suitable for adaptation to another. Here, a prey-grasping motion similar in concept to the strike of a praying mantis in a reptile becomes suitable for modifying into a flapping flight motion.
Additionally, the feathers on the hands and tail have elongated, becoming better suited for helping to sweep prey into the hands.
Next is Caudipteryx:
This species had hand and tail feathers even more developed than the previous species, and longer feathers, more like that of modern birds:
However, it is clear that this was still not a free-flying animal yet, because the forelimbs were too short and the feathers not long enough to support its weight, and the feathers were symmetrical (equal sized "fins" on each side of the central quill). It also had very reduced teeth compared to earlier specimens and a stubby beak:
But the elongation of the feathers indicates some aerodynamic purpose, presumably gliding after leaping (or falling) from trees which it had climbed with its clawed limbs, in the manner of a flying squirrel. Feathers which were developed "for" heat retention and then pressed into service to help scoop prey were now "found" to be useful for breaking falls or gliding to cover distance (or swooping down on prey?).
Next is Sinornithosaurus:
Similar to the preceding species, except that the pubis bone has now shifted to point to the back instead of the front, a key feature in modern birds (when compared to the forward-facing publis bone in reptiles). Here are some of the forearm feathers in detail:
Long feathers in detail:
Artists' reconstruction:
Next is Archaeopteryx:
The transition to flight is now well underway. Archaeopteryx has the reversed hallux (thumb) characteristic of modern birds, and fully developed feathers of the type used for flight (long, aligned with each other, and assymetrical indicating that the feathers have been refined to function aerodynamically). The feathers and limbs are easily long enough to support the weight of this species in flight. However, it lacks some structures which would make endurance flying more practical (such as a keeled sternum for efficient anchoring of the pectoral muscles which power the downstroke) and fused chest vertebrae. Archaeopteryx also retains a number of clearly reptilian features still, including a clawed "hand" emerging from the wings, small reptilian teeth, and a long bony tail. After the previous species' gliding abilities gave it an advantage, evolution would have strongly selected for more improvements in "flying" ability, pushing the species towards something more resembling sustained powered flight.
Next is Confuciusornis:
This species had a nearly modern flight apparatus. It also displays transitional traits between a reptilian grasping "hand" and a fully formed wing as in modern birds -- the outer two digits (the earlier species had three-fingered "hands") in Confuciusornis are still free, but the center digit has now formed flat, broad bones as seen in the wings of modern birds.
Additionally, the foot is now well on its way towards being a perching foot as in modern birds:
It also has a keeled sternum better suited for long flight, and a reduced number of vertebrae in the tail, on its way towards becoming the truncated tail of modern birds (which while prominent, is a small flap of muscle made to look large only because of the long feathers attached).
From this species it's only a small number of minor changes to finish the transition into the modern bird family.
(Hey, who said there are no transitional fossils? Oh, right, a lot of dishonest creationists. And there are a lot more than this, I've just posted some of the more significant milestones.)
There's been a very recent fossil find along this same lineage, too new for me to have found any online images to include in this article. And analysis is still underway to determine exactly where it fits into the above lineage. But it has well-formed feathers, which extend out from both the "arms" and the legs. Although it wasn't advanced enough to fully fly, the balanced feathering on the front and back would have made it ideally suited for gliding like a flying squirrel, and it may be another link between the stage where feathers had not yet been pressed into service as aerodynamic aids, and the time when they began to be used more and more to catch the air and developing towards a "forelimbs as wings" specialization.
So in short, to answer your question about how flight could have developed in birds, the progression is most likely some minor refinement on the following:
1. Scales modified into downy feathers for heat retention.
2. Downy feathers modified into "straight" feathers for better heat retention (modern birds still use their body "contour feathers" in this fashion).
3. Straight feathers modified into a "grasping basket" on the hands (with an accompanying increase in reach for the same purpose).
4. Long limbs with long feathers refined to better survive falls to the ground.
5. "Parachute" feathers refined for better control, leading to gliding.
6. Gliding refined into better controlled, longer gliding.
7. Long gliding refined into short powered "hops".
8. Short powered flight refined into longer powered flight.
9. Longer powered flight refined into long-distance flying.Note that in each stage, the current configuration has already set the stage for natural selection to "prefer" individuals which better meet the requirements of the next stage. Evolution most often works like this; by taking some pre-existing ability or structure, and finding a better use for it or a better way to make it perform its current use.
Fish to elephant evolutionary transition
Tell me, of any two consecutive fossils in the following list, do any differ so much from each other that anti-evolutionists wouldn't just write it off as "just adaptation", or "just microevolution"? [All of the listed specimens are actual fossils]
(Most of the above text is from The Transitional Vertebrate Fossils FAQ, and is the result of hard work by Kathleen Hunt, who deserves the credit. I've just extracted the relevant individual portions and assembled them into one direct fish-to-elephant sequence.) If you like that, here are a few hundred more.Fish to Amphibian transition: 1. Cheirolepis, (early Devonian, 400 million years ago) -- Primitive bony ray-finned fishes that gave rise to the vast majority of living fish. Heavy acanthodian-type scales, acanthodian-like skull, and big notocord.
2. Osteolepis (mid-Devonian, 390 million years ago) -- One of the earliest crossopterygian lobe-finned fishes, still sharing some characters with the lungfish (the other lobe-finned fishes). Had paired fins with a leg-like arrangement of major limb bones, capable of flexing at the "elbow", and had an early-amphibian-like skull and teeth.
3. Eusthenopteron, Sterropterygion (mid-late Devonian, 380 million years ago) -- Early rhipidistian lobe-finned fish roughly intermediate between early crossopterygian fish and the earliest amphibians. Skull very amphibian-like. Strong amphibian- like backbone. Fins very like early amphibian feet in the overall layout of the major bones, muscle attachments, and bone processes, with tetrapod-like tetrahedral humerus, and tetrapod-like elbow and knee joints. But there are no perceptible "toes", just a set of identical fin rays. Body & skull proportions rather fishlike.
4. Panderichthys, Elpistostege (mid-late Devonian, about 370 Mya) -- These "panderichthyids" are very tetrapod-like lobe-finned fish. Unlike Eusthenopteron, these fish actually look like tetrapods in overall proportions (flattened bodies, dorsally placed orbits, frontal bones! in the skull, straight tails, etc.) and have remarkably foot-like fins.
5. Obruchevichthys(middle Late Devonian, about 370 Mya -- Discovered in 1991 in Scotland, these are the earliest known tetrapod remains. The humerus is mostly tetrapod-like but retains some fish features. The discoverer, Ahlberg (1991), said: "It [the humerus] is more tetrapod-like than any fish humerus, but lacks the characteristic early tetrapod 'L-shape'...this seems to be a primitive, fish-like character....although the tibia clearly belongs to a leg, the humerus differs enough from the early tetrapod pattern to make it uncertain whether the appendage carried digits or a fin. At first sight the combination of two such extremities in the same animal seems highly unlikely on functional grounds. If, however, tetrapod limbs evolved for aquatic rather than terrestrial locomotion, as recently suggested, such a morphology might be perfectly workable."
6. Hynerpeton, Acanthostega, Ichthyostega (late Devonian, 360 Mya) -- A little later, the fin-to-foot transition was almost complete, and we have a set of early tetrapod fossils that clearly did have feet. The most complete are Ichthyostega, Acanthostega gunnari, and the newly described Hynerpeton bassetti (Daeschler et al., 1994). (There are also other genera known from more fragmentary fossils.) Hynerpeton is the earliest of these three genera (365 Ma), but is more advanced in some ways; the other two genera retained more fish- like characters longer than the Hynerpeton lineage did. Acanthostega still had internal gills, adding further support to the suggestion that unique tetrapod characters such as limbs with digits evolved first for use in water rather than for walking on land. Acanthostega also had a remarkably fish-like shoulder and forelimb. Ichthyostega was also very fishlike, retaining a fish-like finned tail, permanent lateral line system, and notochord. It turns out that Acanthostega's front foot had eight toes, and Ichthyostega's hind foot had seven toes, giving both feet the look of a short, stout flipper with many "toe rays" similar to fin rays. All you have to do to a lobe- fin to make it into a many-toed foot like this is curl it, wrapping the fin rays forward around the end of the limb. In fact, this is exactly how feet develop in larval amphibians, from a curled limb bud. Hynerpeton, in contrast, probably did not have internal gills and already had a well-developed shoulder girdle; it could elevate and retract its forelimb strongly, and it had strong muscles that attached the shoulder to the rest of the body (Daeschler et al., 1994).
7. Labyrinthodonts (eg Pholidogaster, Pteroplax) (late Dev./early Miss., 355 Mya) -- These larger amphibians still have some icthyostegid fish features, such as skull bone patterns, labyrinthine tooth dentine, presence & pattern of large palatal tusks, the fish skull hinge, pieces of gill structure between cheek & shoulder, and the vertebral structure. But they have lost several other fish features: the fin rays in the tail are gone, the vertebrae are stronger and interlocking, the nasal passage for air intake is well defined, etc.
Amphibian to Reptile transition: 8. Pholidogaster (Mississippian, about 330 Ma) -- A group of large labrinthodont amphibians, transitional between the early amphibians (the ichthyostegids, described above) and later amphibians such as rhachitomes and anthracosaurs.
9. Proterogyrinus (late Mississippian, 325 Mya) -- Classic labyrinthodont-amphibian skull and teeth, but with reptilian vertebrae, pelvis, humerus, and digits. Still has fish skull hinge. Amphibian ankle. 5-toed hand and a 2-3-4-5-3 (almost reptilian) phalangeal count.
10. Limnoscelis, Tseajaia (late Carboniferous, 300 Mya) -- Amphibians apparently derived from the early anthracosaurs, but with additional reptilian features: structure of braincase, reptilian jaw muscle, expanded neural arches.
11. Solenodonsaurus (mid-Pennsylvanian) -- An incomplete fossil, apparently between the anthracosaurs and the cotylosaurs. Loss of palatal fangs, loss of lateral line on head, etc. Still just a single sacral vertebra, though.
12. Hylonomus, Paleothyris (early Pennsylvanian) -- These are protorothyrids, very early cotylosaurs (primitive reptiles). They were quite little, lizard-sized animals with amphibian-like skulls (amphibian pineal opening, dermal bone, etc.), shoulder, pelvis, & limbs, and intermediate teeth and vertebrae. Rest of skeleton reptilian, with reptilian jaw muscle, no palatal fangs, and spool-shaped vertebral centra. Probably no eardrum yet.
13. Paleothyris (early Pennsylvanian) -- An early captorhinomorph reptile, with no temporal fenestrae at all.
14. Protoclepsydrops haplous (early Pennsylvanian) -- The earliest known synapsid reptile. Little temporal fenestra, with all surrounding bones intact. Had amphibian-type vertebrae with tiny neural processes. (reptiles had only just separated from the amphibians)
15. Clepsydrops (early Pennsylvanian) -- The second earliest known synapsid.
Reptile to Mammal transition: 16. Archaeothyris (early-mid Pennsylvanian) -- A slightly later ophiacodont. Small temporal fenestra, now with some reduced bones (supratemporal). Braincase still just loosely attached to skull. Slight hint of different tooth types. Still has some extremely primitive, amphibian/captorhinid features in the jaw, foot, and skull. Limbs, posture, etc. typically reptilian, though the ilium (major hip bone) was slightly enlarged.
17. Varanops (early Permian) -- Temporal fenestra further enlarged. Braincase floor shows first mammalian tendencies & first signs of stronger attachment to rest of skull (occiput more strongly attached). Lower jaw shows first changes in jaw musculature (slight coronoid eminence). Body narrower, deeper: vertebral column more strongly constructed. Ilium further enlarged, lower-limb musculature starts to change (prominent fourth trochanter on femur). This animal was more mobile and active. Too late to be a true ancestor, and must be a "cousin".
18. Haptodus (late Pennsylvanian) -- One of the first known sphenacodonts, showing the initiation of sphenacodont features while retaining many primitive features of the ophiacodonts. Occiput still more strongly attached to the braincase. Teeth become size-differentiated, with biggest teeth in canine region and fewer teeth overall. Stronger jaw muscles. Vertebrae parts & joints more mammalian. Neural spines on vertebrae longer. Hip strengthened by fusing to three sacral vertebrae instead of just two. Limbs very well developed.
19. Dimetrodon, Sphenacodon or a similar sphenacodont (late Pennsylvanian to early Permian, 270 Ma) -- More advanced pelycosaurs, clearly closely related to the first therapsids (next). Dimetrodon is almost definitely a "cousin" and not a direct ancestor, but as it is known from very complete fossils, it's a good model for sphenacodont anatomy. Medium-sized fenestra. Teeth further differentiated, with small incisors, two huge deep- rooted upper canines on each side, followed by smaller cheek teeth, all replaced continuously. Fully reptilian jaw hinge. Lower jaw bone made of multiple bones & with first signs of a bony prong later involved in the eardrum, but there was no eardrum yet, so these reptiles could only hear ground-borne vibrations (they did have a reptilian middle ear). Vertebrae had still longer neural spines (spectacularly so in Dimetrodon, which had a sail), and longer transverse spines for stronger locomotion muscles.
20. Biarmosuchia (late Permian) -- A therocephalian -- one of the earliest, most primitive therapsids. Several primitive, sphenacodontid features retained: jaw muscles inside the skull, platelike occiput, palatal teeth. New features: Temporal fenestra further enlarged, occupying virtually all of the cheek, with the supratemporal bone completely gone. Occipital plate slanted slightly backwards rather than forwards as in pelycosaurs, and attached still more strongly to the braincase. Upper jaw bone (maxillary) expanded to separate lacrymal from nasal bones, intermediate between early reptiles and later mammals. Still no secondary palate, but the vomer bones of the palate developed a backward extension below the palatine bones. This is the first step toward a secondary palate, and with exactly the same pattern seen in cynodonts. Canine teeth larger, dominating the dentition. Variable tooth replacement: some therocephalians (e.g Scylacosaurus) had just one canine, like mammals, and stopped replacing the canine after reaching adult size. Jaw hinge more mammalian in position and shape, jaw musculature stronger (especially the mammalian jaw muscle). The amphibian-like hinged upper jaw finally became immovable. Vertebrae still sphenacodontid-like. Radical alteration in the method of locomotion, with a much more mobile forelimb, more upright hindlimb, & more mammalian femur & pelvis. Primitive sphenacodontid humerus. The toes were approaching equal length, as in mammals, with #toe bones varying from reptilian to mammalian. The neck & tail vertebrae became distinctly different from trunk vertebrae. Probably had an eardrum in the lower jaw, by the jaw hinge.
21. Procynosuchus (latest Permian) -- The first known cynodont -- a famous group of very mammal-like therapsid reptiles, sometimes considered to be the first mammals. Probably arose from the therocephalians, judging from the distinctive secondary palate and numerous other skull characters. Enormous temporal fossae for very strong jaw muscles, formed by just one of the reptilian jaw muscles, which has now become the mammalian masseter. The large fossae is now bounded only by the thin zygomatic arch (cheekbone to you & me). Secondary palate now composed mainly of palatine bones (mammalian), rather than vomers and maxilla as in older forms; it's still only a partial bony palate (completed in life with soft tissue). Lower incisor teeth was reduced to four (per side), instead of the previous six (early mammals had three). Dentary now is 3/4 of lower jaw; the other bones are now a small complex near the jaw hinge. Jaw hinge still reptilian. Vertebral column starts to look mammalian: first two vertebrae modified for head movements, and lumbar vertebrae start to lose ribs, the first sign of functional division into thoracic and lumbar regions. Scapula beginning to change shape. Further enlargement of the ilium and reduction of the pubis in the hip. A diaphragm may have been present.
22. Dvinia [also "Permocynodon"] (latest Permian) -- Another early cynodont. First signs of teeth that are more than simple stabbing points -- cheek teeth develop a tiny cusp. The temporal fenestra increased still further. Various changes in the floor of the braincase; enlarged brain. The dentary bone was now the major bone of the lower jaw. The other jaw bones that had been present in early reptiles were reduced to a complex of smaller bones near the jaw hinge. Single occipital condyle splitting into two surfaces. The postcranial skeleton of Dvinia is virtually unknown and it is not therefore certain whether the typical features found at the next level had already evolved by this one. Metabolic rate was probably increased, at least approaching homeothermy.
23. Thrinaxodon (early Triassic) -- A more advanced "galesaurid" cynodont. Further development of several of the cynodont features seen already. Temporal fenestra still larger, larger jaw muscle attachments. Bony secondary palate almost complete. Functional division of teeth: incisors (four uppers and three lowers), canines, and then 7-9 cheek teeth with cusps for chewing. The cheek teeth were all alike, though (no premolars & molars), did not occlude together, were all single- rooted, and were replaced throughout life in alternate waves. Dentary still larger, with the little quadrate and articular bones were loosely attached. The stapes now touched the inner side of the quadrate. First sign of the mammalian jaw hinge, a ligamentous connection between the lower jaw and the squamosal bone of the skull. The occipital condyle is now two slightly separated surfaces, though not separated as far as the mammalian double condyles. Vertebral connections more mammalian, and lumbar ribs reduced. Scapula shows development of a new mammalian shoulder muscle. Ilium increased again, and all four legs fully upright, not sprawling. Tail short, as is necessary for agile quadrupedal locomotion. The whole locomotion was more agile. Number of toe bones is 2.3.4.4.3, intermediate between reptile number (2.3.4.5.4) and mammalian (2.3.3.3.3), and the "extra" toe bones were tiny. Nearly complete skeletons of these animals have been found curled up - a possible reaction to conserve heat, indicating possible endothermy? Adults and juveniles have been found together, possibly a sign of parental care. The specialization of the lumbar area (e.g. reduction of ribs) is indicative of the presence of a diaphragm, needed for higher O2 intake and homeothermy. NOTE on hearing: The eardrum had developed in the only place available for it -- the lower jaw, right near the jaw hinge, supported by a wide prong (reflected lamina) of the angular bone. These animals could now hear airborne sound, transmitted through the eardrum to two small lower jaw bones, the articular and the quadrate, which contacted the stapes in the skull, which contacted the cochlea. Rather a roundabout system and sensitive to low-frequency sound only, but better than no eardrum at all! Cynodonts developed quite loose quadrates and articulars that could vibrate freely for sound transmittal while still functioning as a jaw joint, strengthened by the mammalian jaw joint right next to it. All early mammals from the Lower Jurassic have this low-frequency ear and a double jaw joint. By the middle Jurassic, mammals lost the reptilian joint (though it still occurs briefly in embryos) and the two bones moved into the nearby middle ear, became smaller, and became much more sensitive to high-frequency sounds.
24. Cynognathus (early Triassic, 240 Ma; suspected to have existed even earlier) -- We're now at advanced cynodont level. Temporal fenestra larger. Teeth differentiating further; cheek teeth with cusps met in true occlusion for slicing up food, rate of replacement reduced, with mammalian-style tooth roots (though single roots). Dentary still larger, forming 90% of the muscle-bearing part of the lower jaw. TWO JAW JOINTS in place, mammalian and reptilian: A new bony jaw joint existed between the squamosal (skull) and the surangular bone (lower jaw), while the other jaw joint bones were reduced to a compound rod lying in a trough in the dentary, close to the middle ear. Ribs more mammalian. Scapula halfway to the mammalian condition. Limbs were held under body. There is possible evidence for fur in fossil pawprints.
25. Diademodon (early Triassic, 240 Ma; same strata as Cynognathus) -- Temporal fenestra larger still, for still stronger jaw muscles. True bony secondary palate formed exactly as in mammals, but didn't extend quite as far back. Turbinate bones possibly present in the nose (warm-blooded?). Dental changes continue: rate of tooth replacement had decreased, cheek teeth have better cusps & consistent wear facets (better occlusion). Lower jaw almost entirely dentary, with tiny articular at the hinge. Still a double jaw joint. Ribs shorten suddenly in lumbar region, probably improving diaphragm function & locomotion. Mammalian toe bones (2.3.3.3.3), with closely related species still showing variable numbers.
26. Probelesodon (mid-Triassic; South America) -- Fenestra very large, still separate from eyesocket (with postorbital bar). Secondary palate longer, but still not complete. Teeth double-rooted, as in mammals. Nares separated. Second jaw joint stronger. Lumbar ribs totally lost; thoracic ribs more mammalian, vertebral connections very mammalian. Hip & femur more mammalian.
27. Probainognathus (mid-Triassic, 239-235 Ma, Argentina) -- Larger brain with various skull changes: pineal foramen ("third eye") closes, fusion of some skull plates. Cheekbone slender, low down on the side of the eye socket. Postorbital bar still there. Additional cusps on cheek teeth. Still two jaw joints. Still had cervical ribs & lumbar ribs, but they were very short. Reptilian "costal plates" on thoracic ribs mostly lost. Mammalian #toe bones.
28. Pachygenelus, Diarthrognathus (earliest Jurassic, 209 Ma) -- These are trithelodontids. Inflation of nasal cavity, establishment of Eustachian tubes between ear and pharynx, loss of postorbital bar. Alternate replacement of mostly single- rooted teeth. This group also began to develop double tooth roots -- in Pachygenelus the single root of the cheek teeth begins to split in two at the base. Pachygenelus also has mammalian tooth enamel, and mammalian tooth occlusion. Double jaw joint, with the second joint now a dentary-squamosal (instead of surangular), fully mammalian. Incipient dentary condyle. Reptilian jaw joint still present but functioning almost entirely in hearing; postdentary bones further reduced to tiny rod of bones in jaw near middle ear; probably could hear high frequencies now. More mammalian neck vertebrae for a flexible neck. Hip more mammalian, with a very mammalian iliac blade & femur. Highly mobile, mammalian-style shoulder. Probably had coupled locomotion & breathing.
29. Sinoconodon (early Jurassic, 208 Ma) -- The next known very ancient proto-mammal. Eyesocket fully mammalian now (closed medial wall). Hindbrain expanded. Permanent cheekteeth, like mammals, but the other teeth were still replaced several times. Mammalian jaw joint stronger, with large dentary condyle fitting into a distinct fossa on the squamosal. This final refinement of the joint automatically makes this animal a true "mammal". Reptilian jaw joint still present, though tiny.
Proto-mammal to Placental Mammal transition: 30. Kuehneotherium (early Jurassic, about 205 Ma) -- A slightly later proto-mammal, sometimes considered the first known pantothere (primitive placental-type mammal). Teeth and skull like a placental mammal. The three major cusps on the upper & lower molars were rotated to form interlocking shearing triangles as in the more advanced placental mammals & marsupials. Still has a double jaw joint, though.
31. Eozostrodon, Morganucodon, Haldanodon (early Jurassic, ~205 Ma) -- A group of early proto-mammals called "morganucodonts". The restructuring of the secondary palate and the floor of the braincase had continued, and was now very mammalian. Truly mammalian teeth: the cheek teeth were finally differentiated into simple premolars and more complex molars, and teeth were replaced only once. Triangular- cusped molars. Reversal of the previous trend toward reduced incisors, with lower incisors increasing to four. Tiny remnant of the reptilian jaw joint. Once thought to be ancestral to monotremes only, but now thought to be ancestral to all three groups of modern mammals -- monotremes, marsupials, and placentals.
32. Peramus (late Jurassic, about 155 Ma) -- A "eupantothere" (more advanced placental-type mammal). The closest known relative of the placentals & marsupials. Triconodont molar has with more defined cusps. This fossil is known only from teeth, but judging from closely related eupantotheres (e.g. Amphitherium) it had finally lost the reptilian jaw joint, attaing a fully mammalian three-boned middle ear with excellent high-frequency hearing. Has only 8 cheek teeth, less than other eupantotheres and close to the 7 of the first placental mammals. Also has a large talonid on its "tribosphenic" molars, almost as large as that of the first placentals -- the first development of grinding capability.
33. Endotherium (very latest Jurassic, 147 Ma) -- An advanced eupantothere. Fully tribosphenic molars with a well- developed talonid. Known only from one specimen. From Asia; recent fossil finds in Asia suggest that the tribosphenic molar evolved there.
34. Vincelestes neuquenianus (early Cretaceous, 135 Ma) -- A probably-placental mammal with some marsupial traits, known from some nice skulls. Placental-type braincase and coiled cochlea. Its intracranial arteries & veins ran in a composite monotreme/placental pattern derived from homologous extracranial vessels in the cynodonts. (Rougier et al., 1992)
35. Kennalestes and Asioryctes (late Cretaceous, Mongolia) -- Small, slender animals; eyesocket open behind; simple ring to support eardrum; primitive placental-type brain with large olfactory bulbs; basic primitive tribosphenic tooth pattern. Canine now double rooted. Still just a trace of a non-dentary bone, the coronoid, on the otherwise all-dentary jaw. "Could have given rise to nearly all subsequent placentals." says Carroll (1988).
Placental mammal to elephant transition: 36. Protungulatum (latest Cretaceous) -- Transitional between earliest placental mammals and the condylarths (primitive, small hoofed animals). These early, simple insectivore- like small mammals had one new development: their cheek teeth had grinding surfaces instead of simple, pointed cusps. They were the first mammal herbivores. All their other features are generalized and primitive -- simple plantigrade five-toed clawed feet, all teeth present (3:1:4:3) with no gaps, all limb bones present and unfused, pointy-faced, narrow small brain, eyesocket not closed.
37. Minchenella or a similar condylarth (late Paleocene) -- Known only from lower jaws. Has a distinctive broadened shelf on the third molar.
38. Phenacolophus (late Paleocene or early Eocene) -- An early embrithopod (very early, slightly elephant-like condylarths), thought to be the stem-group of all elephants.
39. Pilgrimella (early Eocene) -- An anthracobunid (early proto-elephant condylarth), with massive molar cusps aligned in two transverse ridges.
40. Unnamed species of proto-elephant (early Eocene) -- Discovered recently in Algeria. Had slightly enlarged upper incisors (the beginnings of tusks), and various tooth reductions. Still had "normal" molars instead of the strange multi-layered molars of modern elephants. Had the high forehead and pneumatized skull bones of later elephants, and was clearly a heavy-boned, slow animal. Only one meter tall.
41. Moeritherium, Numidotherium, Barytherium (early-mid Eocene) -- A group of three similar very early elephants. It is unclear which of the three came first. Pig-sized with stout legs, broad spreading feet and flat hooves. Elephantish face with the eye set far forward & a very deep jaw. Second incisors enlarged into short tusks, in upper and lower jaws; little first incisors still present; loss of some teeth. No trunk.
42. Paleomastodon, Phiomia (early Oligocene) -- The first "mastodonts", a medium-sized animals with a trunk, long lower jaws, and short upper and lower tusks. Lost first incisors and canines. Molars still have heavy rounded cusps, with enamel bands becoming irregular. Phiomia was up to eight feet tall.
43. Gomphotherium (early Miocene) -- Basically a large edition of Phiomia, with tooth enamel bands becoming very irregular. Two long rows cusps on teeth became cross- crests when worn down. Gave rise to several families of elephant- relatives that spread all over the world. From here on the elephant lineages are known to the species level.
44a. The mastodon lineage split off here, becoming more adapted to a forest browser niche, and going through Miomastodon (Miocene) and Pliomastodon (Pliocene), to Mastodon (or "Mammut", Pleistocene).
44b. Meanwhile, the elephant lineage became still larger, adapting to a savannah/steppe grazer niche:
45. Stegotetrabelodon (late Miocene) -- One of the first of the "true" elephants, but still had two long rows of cross-crests, functional premolars, and lower tusks. Other early Miocene genera show compression of the molar cusps into plates (a modern feature ), with exactly as many plates as there were cusps. Molars start erupting from front to back, actually moving forward in the jaw throughout life.
46. Primelephas (latest Miocene) -- Short lower jaw makes it look like an elephant now. Reduction & loss of premolars. Very numerous plates on the molars, now; we're now at the modern elephants' bizarre system of one enormous multi-layered molar being functional at a time, moving forward in the jaw.
47. Primelephas gomphotheroides (mid-Pliocene) -- A later species that split into three lineages, Loxodonta, Elephas, and Mammuthus:
The Pleistocene record for elephants is very good. In general, after the earliest forms of the three modern genera appeared, they show very smooth, continuous evolution with almost half of the speciation events preserved in fossils. For instance, Carroll (1988) says: "Within the genus Elephas, species demonstrate continuous change over a period of 4.5 million years. ...the elephants provide excellent evidence of significant morphological change within species, through species within genera, and through genera within a family...."
- Loxodonta adaurora (5 Ma). Gave rise to the modern African elephant Loxodonta africana about 3.5 Ma.
- Elephas ekorensis (5 Ma), an early Asian elephant with rather primitive molars, clearly derived directly from P. gomphotheroides. Led directly to:
- Elephas recki, which sent off one side branch, E. hydrusicus, at 3.8 Ma, and then continued changing on its own until it became E. iolensis.
- Elephas maximus, the modern Asian elephant, clearly derived from
- E. hysudricus. Strikingly similar to young E. hysudricus animals. Possibly a case of neoteny (in which "new" traits are simply juvenile features retained into adulthood).
- Mammuthus meridionalis, clearly derived from P. gomphotheroides. Spread around the northern hemisphere. In Europe, led to M. armeniacus/trogontherii, and then to M. primigenius. In North America, led to M. imperator and then M. columbi.
Species-species transitions among the elephants:
- Maglio (1973) studied Pleistocene elephants closely. Overall, Maglio showed that at least 7 of the 17 Quaternary elephant species arose through smooth anagenesis transitions from their ancestors. For example, he said that Elephas recki "can be traced through a progressive series of stages...These stages pass almost imperceptibly into each other....In the late Pleistocene a more progressive elephant appears which I retain as a distinct species, E. iolensis, only as a matter of convenience. Although as a group, material referred to E. iolensis is distinct from that of E. recki, some intermediate specimens are known, and E. iolensis seems to represent a very progressive, terminal stage in the E. recki specific lineage."
- Maglio also documented very smooth transitions between three Eurasian mammoth species: Mammuthus meridionalis --> M. armeniacus (or M. trogontherii) --> M. primigenius.
- Lister (1993) reanalyzed mammoth teeth and confirmed Maglio's scheme of gradual evolution in European mammoths, and found evidence for gradual transitions in the North American mammoths too.
Similar fossil sequences can be listed for the majority of other major-group transitions.
(Did I hear a creationist in the back row say something about "no transitional fossils?")
Note that the changes between any two sequential transitionals are small enough that most creationists would write them off as only "microevolution" -- and yet those 50-or-so "microevolutionary" steps turn a fish into an elephant, which even the most stubborn creationist would have to concede is "macroevolution".
Once you've answered the first question, here's a second one for you: If evolutionary common descent *hasn't* actually happened -- if the different animal "kinds" were just *poofed* into existence fully-formed -- then why is it possible to order known fossils into such a smooth "transitional" chain *at all*, in a way that makes sense and is chronologically, morphologically, genetically, paleontologically, geographically (etc. etc.) consistent with the (allegedly) "non-existent" evolutionary transitions? And no, it's not possible to assemble a sequence of fossils in just any damned order you want, so don't try *that* excuse -- even evolutionary biologists aren't capable of putting together a transitional fossil sequence "showing", say, a cat evolving into a bird, or a butterfly into a bat. Please explain.
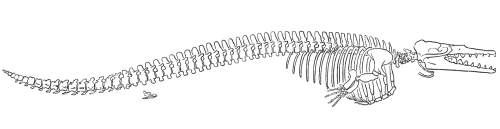
Whale Evolution
The transitional fossils in the evolutionary origin of whales is especially striking. The following is an excerpt from The Origin of Whales and the Power of Independent Evidence . This excerpt is excellent all by itself, but one should really read the entire essay in order to get the "big picture" of whale evolution:The evidenceThe paleontological (i.e. fossil) evidence for evolutionary transitions is overwhelming to anyone who has actually examined the evidence with an open mind. However, a stubborn person attempting to deny the obvious can rationalize it away by refusing to see the clear sequences of morphological change, and insisting that one can't "prove" that the various fossil specimens are "really" necessarily related. That excuse crumbles when one compares the fossil evidence to the *many* other independent lines of evidence which confirm the fossil evidence. For example, concerning whale evolution:
The evidence that whales descended from terrestrial mammals is here divided into nine independent parts: paleontological, morphological, molecular biological, vestigial, embryological, geochemical, paleoenvironmental, paleobiogeographical, and chronological. Although my summary of the evidence is not exhaustive, it shows that the current view of whale evolution is supported by scientific research in several distinct disciplines.
1. Paleontological evidence
The paleontological evidence comes from studying the fossil sequence from terrestrial mammals through more and more whale-like forms until the appearance of modern whales. Although the early whales (Archaeocetes) exhibit greater diversity than I have space to discuss here, the examples in this section represent the trends that we see in this taxon. Although there are two modern suborders of whales (Odontocetes and Mysticetes), this discussion will focus on the origin of the whales as an order of mammals, and set aside the issues related to the diversification into suborders.
Sinonyx
We start with Sinonyx, a wolf-sized mesonychid (a primitive ungulate from the order Condylarthra, which gave rise to artiodactyls, perissodactyls, proboscideans, and so on) from the late Paleocene, about 60 million years ago. The characters that link Sinonyx to the whales, thus indicating that they are relatives, include an elongated muzzle, an enlarged jugular foramen, and a short basicranium (Zhou and others 1995). The tooth count was the primitive mammalian number (44); the teeth were differentiated as are the heterodont teeth of today's mammals. The molars were very narrow shearing teeth, especially in the lower jaw, but possessed multiple cusps. The elongation of the muzzle is often associated with hunting fish - all fish-hunting whales, as well as dolphins, have elongated muzzles. These features were atypical of mesonychids, indicating that Sinonyx was already developing the adaptations that later became the basis of the whales' specialized way of life.
Pakicetus
The next fossil in the sequence, Pakicetus, is the oldest cetacean, and the first known archaeocete. It is from the early Eocene of Pakistan, about 52 million years ago (Gingerich and others 1983). Although it is known only from fragmentary skull remains, those remains are very diagnostic, and they are definitely intermediate between Sinonyxand later whales. This is especially the case for the teeth. The upper and lower molars, which have multiple cusps, are still similar to those of Sinonyx, but the premolars have become simple triangular teeth composed of a single cusp serrated on its front and back edges. The teeth of later whales show even more simplification into simple serrated triangles, like those of carnivorous sharks, indicating that Pakicetus's teeth were adapted to hunting fish.
Gingrich and others (1983) published this reconstruction of the skull of
Pakicetus inachus (redrawn for RNCSE by Janet Dreyer).
A well-preserved cranium shows that Pakicetus was definitely a cetacean with a narrow braincase, a high, narrow sagittal crest, and prominent lambdoidal crests. Gingerich and others (1983) reconstructed a composite skull that was about 35 centimeters long. Pakicetus did not hear well underwater. Its skull had neither dense tympanic bullae nor sinuses isolating the left auditory area from the right one - an adaptation of later whales that allows directional hearing under water and prevents transmission of sounds through the skull (Gingerich and others 1983). All living whales have foam-filled sinuses along with dense tympanic bullae that create an impedance contrast so they can separate sounds arriving from different directions. There is also no evidence in Pakicetus of vascularization of the middle ear, which is necessary to regulate the pressure within the middle ear during diving (Gingerich and others 1983). Therefore, Pakicetus was probably incapable of achieving dives of any significant depth. This paleontological assessment of the ecological niche of Pakicetus is entirely consistent with the geochemical and paleoenvironmental evidence. When it came to hearing, Pakicetus was more terrestrial than aquatic, but the shape of its skull was definitely cetacean, and its teeth were between the ancestral and modern states.
Zhou and others (1995) published this reconstruction of the skull of
Sinonyx jiashanensis (redrawn for RNCSE by Janet Dreyer).
Ambulocetus
In the same area that Pakicetus was found, but in sediments about 120 meters higher, Thewissen and colleagues (1994) discovered Ambulocetus natans, "the walking whale that swims", in 1992. Dating from the early to middle Eocene, about 50 million years ago, Ambulocetus is a truly amazing fossil. It was clearly a cetacean, but it also had functional legs and a skeleton that still allowed some degree of terrestrial walking. The conclusion that Ambulocetus could walk by using the hind limbs is supported by its having a large, stout femur. However, because the femur did not have the requisite large attachment points for walking muscles, it could not have been a very efficient walker. Probably it could walk only in the way that modern sea lions can walk - by rotating the hind feet forward and waddling along the ground with the assistance of their forefeet and spinal flexion. When walking, its huge front feet must have pointed laterally to a fair degree since, if they had pointed forward, they would have interfered with each other.
The forelimbs were also intermediate in both structure and function. The ulna and the radius were strong and capable of carrying the weight of the animal on land. The strong elbow was strong but it was inclined rearward, making possible rearward thrusts of the forearm for swimming. However, the wrists, unlike those of modern whales, were flexible.
It is obvious from the anatomy of the spinal column that Ambulocetus must have swum with its spine swaying up and down, propelled by its back feet, oriented to the rear. As with other aquatic mammals using this method of swimming, the back feet were quite large. Unusually, the toes of the back feet terminated in hooves, thus advertising the ungulate ancestry of the animal. The only tail vertebra found is long, making it likely that the tail was also long. The cervical vertebrae were relatively long, compared to those of modern whales; Ambulocetus must have had a flexible neck.
Ambulocetus's skull was quite cetacean (Novacek 1994). It had a long muzzle, teeth that were very similar to later archaeocetes, a reduced zygomatic arch, and a tympanic bulla (which supports the eardrum) that was poorly attached to the skull. Although Ambulocetus apparently lacked a blowhole, the other skull features qualify Ambulocetus as a cetacean. The post-cranial features are clearly in transitional adaptation to the aquatic environment. Thus Ambulocetus is best described as an amphibious, sea-lion-sized fish-eater that was not yet totally disconnected from the terrestrial life of its ancestors.
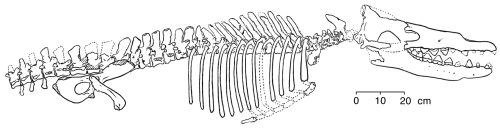
Rodhocetus
In the middle Eocene (46-7 million years ago) Rodhocetus took all of these changes even further, yet still retained a number of primitive terrestrial features (Gingerich and others 1994). It is the earliest archaeocete of which all of the thoracic, lumbar, and sacral vertebrae have been preserved. The lumbar vertebrae had higher neural spines than in earlier whales. The size of these extensions on the top of the vertebrae where muscles are attached indicate that Rodhocetus had developed a powerful tail for swimming.
Gingrich and others (1994) published this reconstruction of the skeleton of
Rodhocetus kasrani (redrawn for RNCSE by Janet Dreyer).
Elsewhere along the spine, the four large sacral vertebrae were unfused. This gave the spine more flexibility and allowed a more powerful thrust while swimming. It is also likely that Rodhocetus had a tail fluke, although such a feature is not preserved in the known fossils: it possessed features - shortened cervical vertebrae, heavy and robust proximal tail vertebrae, and large dorsal spines on the lumbar vertebrae for large tail and other axial muscle attachments - that are associated in modern whales with the development and use of tail flukes. All in all, Rodhocetus must have been a very good tail-swimmer, and it is the earliest fossil whale committed to this manner of swimming.
The pelvis of Rodhocetus was smaller than that of its predecessors, but it was still connected to the sacral vertebrae, meaning that Rodhocetus could still walk on land to some degree. However, the ilium of the pelvis was short compared to that of the mesonychids, making for a less powerful muscular thrust from the hip during walking, and the femur was about 1/3 shorter than Ambulocetus’s, so Rodhocetus probably could not get around as well on land as its predecessors (Gingerich and others 1994).
Rodhocetus's skull was rather large compared to the rest of the skeleton. The premaxillae and dentaries had extended forward even more than its predecessors’, elongating the skull and making it even more cetacean. The molars have higher crowns than in earlier whales and are greatly simplified. The lower molars are higher than they are wide. There is a reduced differentiation among the teeth. For the first time, the nostrils have moved back along the snout and are located above the canine teeth, showing blowhole evolution. The auditory bullae are large and made of dense bone (characteristics unique to cetaceans), but they apparently did not contain the sinuses typical of later whales, making it questionable whether Rodhocetus possessed directional hearing underwater.
Overall, Rodhocetus showed improvements over earlier whales by virtue of its deep, slim thorax, longer head, greater vertebral flexibility, and expanded tail-related musculature. The increase in flexibility and strength in the back and tail with the accompanying decrease in the strength and size of the limbs indicated that it was a good tail-swimmer with a reduced ability to walk on land.
Basilosaurus
The particularly well-known fossil whale Basilosaurus represents the next evolutionary grade in whale evolution (Gingerich 1994). It lived during the late Eocene and latest part of the middle Eocene (35-45 million years ago). Basilosaurus was a long, thin, serpentine animal that was originally thought to have been the remains of a sea serpent (hence it is name, which actually means "king lizard"). Its extreme body length (about 15 meters) appears to be due to a feature unique among whales; its 67 vertebrae are so long compared to other whales of the time and to modern whales that it probably represents a specialization that sets it apart from the lineage that gave rise to modern whales.
What makes Basilosaurus a particularly interesting whale, however, is the distinctive anatomy of its hind limbs (Gingerich and others 1990). It had a nearly complete pelvic girdle and set of hindlimb bones. The limbs were too small for effective propulsion, less than 60 cm long on this 15-meter-long animal, and the pelvic girdle was completely isolated from the spine so that weight-bearing was impossible. Reconstructions of the animal have placed its legs external to the body - a configuration that would represent an important intermediate form in whale evolution.
Although no tail fluke has ever been found (since tail flukes contain no bones and are unlikely to fossilize), Gingerich and others (1990) noted that Basilosaurus's vertebral column shares characteristics of whales that do have tail flukes. The tail and cervical vertebrae are shorter than those of the thoracic and lumbar regions, and Gingerich and others (1990) take these vertebral proportions as evidence that Basilosaurus probably also had a tail fluke.
Further evidence that Basilosaurus spent most of its time in the water comes from another important change in the skull. This animal had a large single nostril that had migrated a short distance back to a point corresponding to the back third of the dental array. The movement from the forward extreme of the snout to the a position nearer the top of the head is characteristic of only those mammals that live in marine or aquatic environments.
Dorudon
Dorudon was a contemporary of Basilosaurus in the late Eocene (about 40 million years ago) and probably represents the group most likely to be ancestral to modern whales (Gingerich 1994). Dorudon lacked the elongated vertebrae of Basilosaurus and was much smaller (about 4-5 meters in length). Dorudon’s dentition was similar to Basilosaurus’s; its cranium, compared to the skulls of Basilosaurus and the previous whales, was somewhat vaulted (Kellogg 1936). Dorudon also did not yet have the skull anatomy that indicates the presence of the apparatus necessary for echolocation (Barnes 1984).
Gingrich and Uhen (1996) published this reconstruction of the skeleton of
Dorudon atrox (redrawn for RNCSE by Janet Dreyer).
Basilosaurus and Dorudon were fully aquatic whales (like Basilosaurus, Dorudon had very small hind limbs that may have projected slightly beyond the body wall). They were no longer tied to the land; in fact, they would not have been able to move around on land at all. Their size and their lack of limbs that could support their weight made them obligate aquatic mammals, a trend that is elaborated and reinforced by subsequent whale taxa.
Clearly, even if we look only at the paleontological evidence, the creationist claim of "No fossil intermediates!" is wrong. In fact, in the case of whales, we have several, beautifully arranged in morphological and chronological order.
In summarizing the paleontological evidence, we have noted the consistent changes that indicate a series of adaptations from more terrestrial to more aquatic environments as we move from the most ancestral to the most recent species. These changes affect the shape of the skull, the shape of the teeth, the position of the nostrils, the size and structure of both the forelimbs and the hindlimbs, the size and shape of the tail, and the structure of the middle ear as it relates to directional hearing underwater and diving. The paleontological evidence records a history of increasing adaptation to life in the water - not just to any way of life in the water, but to life as lived by contemporary whales.
So, what was that you were claiming about Archaeopteryx being the "only" transitional fossil? Would you care to revise your BS claim?Evolution of whales from terrestrial mammals
(From Plagiarized Errors and Molecular Genetics)That's just a quick layman-level overview of *one* of the many ways that whale evolution has been verified. For more technical examinations along several independent lines of evidence, see for example:.
A particularly impressive example of shared retroposons has recently been reported linking cetaceans (whales, dolphins and porpoises) to ruminants and hippopotamuses, and it is instructive to consider this example in some detail. Cetaceans are sea-living animals that bear important similarities to land-living mammals; in particular, the females have mammary glands and nurse their young. Scientists studying mammalian anatomy and physiology have demonstrated greatest similarities between cetaceans and the mammalian group known as artiodactyls (even-toed ungulates) including cows, sheep, camels and pigs. These observations have led to the evolutionist view that whales evolved from a four-legged artiodactyl ancestor that lived on land. Creationists have capitalized on the obvious differences between the familiar artiodactyls and whales, and have ridiculed the idea that whales could have had four-legged land-living ancestors. Creationists who claim that cetaceans did not arise from four-legged land mammals must ignore or somehow dismiss the fossil evidence of apparent whale ancestors looking exactly like one would predict for transitional species between land mammals and whales--with diminutive legs and with ear structures intermediate between those of modern artiodactyls and cetaceans (Nature 368:844,1994; Science 263: 210, 1994). (A discussion of fossil ancestral whale species with references may be found at http://www.talkorigins.org/faqs/faq-transitional/part2b.html#ceta) Creationists must also ignore or dismiss the evidence showing the great similarity between cetacean and artiodactyl gene sequences (Molecular Biology & Evolution 11:357, 1994; ibid 13: 954, 1996; Gatesy et al, Systematic Biology 48:6, 1999).
Recently retroposon evidence has solidified the evolutionary relationship between whales and artiodactyls. Shimamura et al. (Nature 388:666, 1997; Mol Biol Evol 16: 1046, 1999; see also Lum et al., Mol Biol Evol 17:1417, 2000; Nikaido and Okada, Mamm Genome 11:1123, 2000) studied SINE sequences that are highly reduplicated in the DNA of all cetacean species examined. These SINES were also found to be present in the DNA of ruminants (including cows and sheep) but not in DNA of camels and pigs or more distantly related mammals such as horse, elephant, cat, human or kangaroo. These SINES apparently originated in a specific branch of ancestral artiodactyls after this branch diverged from camels, pigs and other mammals, but before the divergence of the lines leading to modern cetaceans, hippopotamus and ruminants. (See Figure 5.) In support of this scenario, Shimamura et al. identified two specific insertions of these SINES in whale DNA (insertions B and C in Figure 5) and showed that in DNA of hippopotamus, cow and sheep these same two sites contained the SINES; but in camel and pig DNA the same sites were "empty" of insertions. More recently, hippopotamus has been identified as the closest living terrestrial relative of cetaceans since hippos and whales share retroposon insertions (illustrated by D and E in Figure 5) that are not found in any other artiodactyls (Nikaido et al, PNAS 96:10261, 1999). The close hippo-whale relationship is consistent with previously reported sequence similarity comparisons (Gatesy, Mol Biol Evol 14:537, 1997) and with recent fossil finds (Gingerich et al., Science 293:2239, 2001; Thewissen et al., Nature 413:277, 2001) that resolve earlier paleontological conflicts with the close whale-hippo relationship. (Some readers have wondered: if ruminants are more closely related to whales than to pigs and camels, why are ruminants anatomically more similar to pigs and camels than they are to whales? Apparently this results from the fact that ruminants, pigs and camels changed relatively little since their last common ancestor, while the cetacean lineage changed dramatically in adapting to an aquatic lifestyle, thereby obliterating many of the features -- like hooves, fur and hind legs -- that are shared between its close ruminant relatives and the more distantly related pigs and camels. This scenario illustrates the fact that the rapid evolutionary development of adaptations to a new niche can occur through key functional mutations, leaving the bulk of the DNA relatively unchanged. The particularly close relationship between whales and hippos is consistent with several shared adaptations to aquatic life, including use of underwater vocalizations for communication and the absence of hair and sebaceous glands.) Thus, retroposon evidence strongly supports the derivation of whales from a common ancestor of hippopotamus and ruminants, consistent with the evolutionary interpretation of fossils and overall DNA sequence similarities. Indeed, the logic of the evidence from shared SINEs is so powerful that SINEs may be the best available characters for deducing species relatedness (Shedlock and Okada, Bioessays 22:148, 2000), even if they are not perfect (Myamoto, Curr. Biology 9:R816, 1999).
Figure 5. Specific SINE insertions can act as "tracers" that illuminate phylogenetic relationships. This figure summarizes some of the data on SINEs found in living artiodactyls and shows how the shared insertions can be interpreted in relation to evolutionary branching. A specific SINE insertion event ("A" in the Figure) apparently occurred in a primitive common ancestor of pigs, ruminants, hippopotamus and cetaceans, since this insertion is present in these modern descendants of that common ancestor; but it is absent in camels, which split off from the other species before this SINE inserted. More recent insertions B and C are present only in ruminants, hippopotamus and cetaceans. Insertions D and E are shared only by hippopotamus and cetaceans, thereby identifying hippopotamus as the closest living relative of cetaceans (at least among the species examined in these studies). SINE insertions F and G occurred in the ruminant lineage after it diverged from the other species; and insertions H and I occurred after divergence of the cetacean lineage.
SINE Evolution, Missing Data, and the Origin of WhalesAnd much, much more.Evidence from Milk Casein Genes that Cetaceans are Close Relatives of Hippopotamid Artiodactyls
Analyses of mitochondrial genomes strongly support a hippopotamus±whale clade
A new Eocene archaeocete (Mammalia, Cetacea) from India and the time of origin of whales
Mysticete (Baleen Whale) Relationships Based upon the Sequence of the Common Cetacean DNA Satellite1
Eocene evolution of whale hearing
Novel Phylogeny of Whales Revisited but Not Revised
New Morphological Evidence for the Phylogeny of Artiodactyla, Cetacea, and Mesonychidae
But the real zinger is how complex even the simplest life forms are.
That's not a "zinger" at all, since evolutionary processes are provably capable of producing arbitrary levels of complexity. You might want to actually go study information science before you again make the error of mistaking your uninformed presumptions for facts.
To randomly create even the simplest life form would be, as some say, like having a tornado go through a junkyard and produce a 747. Not in a billion billion billion years. Forget it, no way, nada, yah right.
If evolutionary processes -- or biological processes in general -- were anything like "a tornado in a junkyard", you might have a valid point. But since they aren't, you don't. Hoyle's goofy "tornado making a 747" analogy was recognized as fatally flawed way back when he made it. What excuse to creationists have for still using it?
Hint: Evolutionary change is the result of the interplay of *three* separate processes. (Extra credit for the creationists: Do you understand even the *basics* of evolution well enough to name them, or are you entirely ignorant of the fundamentals of the field you presume to "lecture" other people about?) Hoyle's cartoonish example models only *one* of them, and leaves out the other two critical components of evolution, and as such is a completely invalid analogy for what evolution can actually do. One has to wonder whether he blew his example out of gross ignorance of biological processes, or through intentional dishonesty, but neither option inspires confidence. The same question arises about the myriad of creationists who repeat his invalid example. For example, what's *your* excuse for presenting such a childishly incorrect argument, which in no way constitutes a valid analogy for evolutionary processes? Are you presenting dishonest sophistry that you *know* is invalid and misleading? Or are you presenting it because you're so ignorant of even the very simplest basics of evolutionary processes that you actually thought that wind through a junkyard was in any way a conceptually accurate model of how evolution actually proceeds?
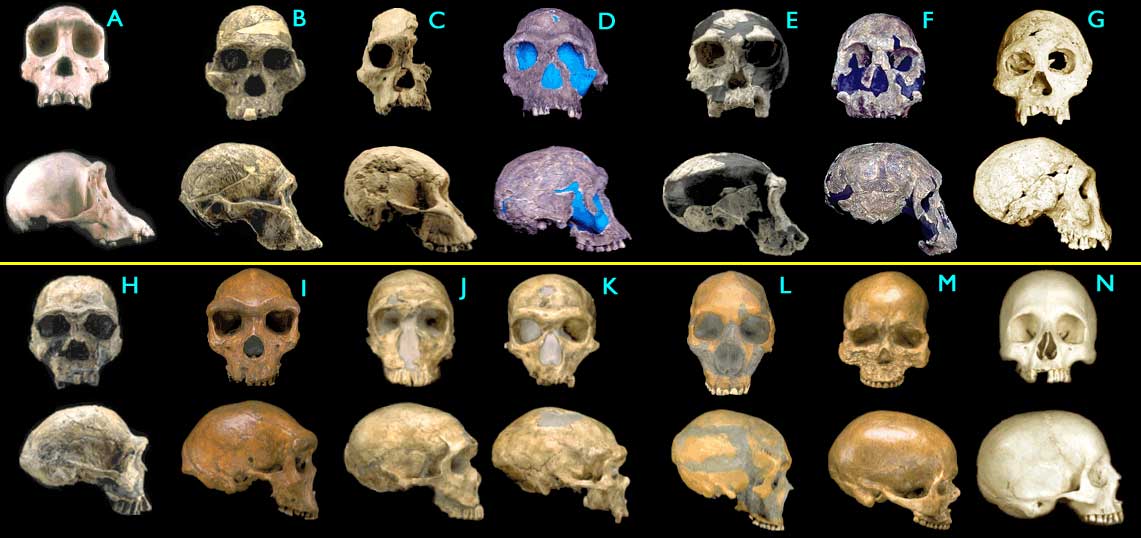
![[Figure1.4.1 (cartoon of vertebrate jaws)]](http://www.talkorigins.org/faqs/comdesc/images/jaws2.gif)
![[Figure1.4.2a (cartoon of vertebrate ears)]](http://www.talkorigins.org/faqs/comdesc/images/reptile_ear.gif)
![[Figure1.4.2b (cartoon of vertebrate ears)]](http://www.talkorigins.org/faqs/comdesc/images/human_ear.gif)
![[Figure1.4.3 (cartoon of vertebrate jaws)]](http://www.talkorigins.org/faqs/comdesc/images/jaws1.gif)
![[Fig. 1a]](http://www.gcssepm.org/images/fossil_a.gif)