Skip to comments.
2003 Antarctic Ozone Hole Equals Record Size
Space Daily ^
| 09/17/2003
Posted on 09/17/2003 10:31:26 AM PDT by cogitator
Antarctic Ozone Hole Roars Back
Measurements over and near Antarctica show that ozone is decreasing more rapidly this year than in previous years and that the size of the ozone hole is now as large as the all time record size of 28 million sq. km during September 2000. This is in stark contrast to the ozone hole last year when it was the smallest in more than a decade after splitting in two during late September.
In recent years, the ozone hole is at or near its maximum size during mid-September, with the maximum sometimes reached in late September. It cannot be predicted with certainty whether the ozone hole will continue to grow during the next few weeks.
Recent variations in size, depth and persistence of the ozone hole are due to year-to-year changes in meteorological conditions in the lower stratosphere over Antarctica, rather than changes in the amount of ozone depleting chemicals present in the ozone layer.
The use of ozone depleting chemicals is presently being controlled through the enforcement of international agreements. Measurements show that most of these chemicals are decreasing in the lower atmosphere and they appear to have reached their peak in the critically important ozone layer in the stratosphere.
There is a delay in the cleansing of these chemicals from the ozone layer, and it is expected to require decades before the stratosphere returns to pre-ozone hole conditions. Complete recovery of the ozone layer will require continuing diligence with the enforcement of the international agreements.
In recognition of the importance of international co-operation on environmental issues and to commemorate the date of the signing of the Montreal Protocol in 1987, 16 September has been designated by the United Nations as International Day for the Preservation of the Ozone Layer.
On this occasion, Prof. G.O.P. Obasi, Secretary-General of the World Meteorological Organization urged all nations to pursue their efforts in the monitoring of the chemical composition of the atmosphere and in the implementation of the Vienna Convention on the Protection of the Ozone Layer and the Montreal Protocol and Amendments on Substances that Deplete the Ozone Layer.
TOPICS: Culture/Society; Foreign Affairs; Front Page News; Government
KEYWORDS: antarctica; cfcs; environment; ozone; ozonehole; ultraviolet
Navigation: use the links below to view more comments.
first previous 1-20, 21-40, 41-60, 61-80, 81-82 next last
To: saminfl
Diffusion means it is spread out or not concentrated. Still, you and your ilk are trying to say that the entire amout of CFCs leaked is concentrated in the upper atmosphere. Do you agree that CFCs are heavier than air? If so, would you not agree that they would be diffused mostly at or near ground level? I can direct you (In fact I think I alrady have) to a web site that refutes what your web site says.Sorry for the hurricane-induced delay in replying, Sam. You've got a major conceptual error in the above paragraph. NOBODY is saying that the entire amount of CFCs leaked is concentrated in the upper atmosphere. Only a very small amount of CFC molecules reach the upper atmosphere (compared to the amount released)! But in the upper atmosphere the CFC molecules are broken down by energetic reactions (cosmic rays, free oxygen), which releases the chlorine and fluorine, which are highly reactive. Cl and F react with ozone, breaking it down. In the normal stratosphere, this has resulted in a slight (5%) ozone concentration reduction. But in the polar vortex, ozone breakdown reactions are catalyzed on polar ice cloud particles, resulting in a much larger decrease in CFC concentration.
Does your Web site refute that scenario? Or does it merely refute erroneous models?
To: cogitator
OZONE DEPLETION AND CFC THEORY by S. Fred Singer Science, September 1993
1. Gary Taubes' article ("The Ozone Backlash," Science, June 11, pp 1580-1583) refers to my commentaries as "purporting to shoot holes in the [CFC] theory of ozone depletion." This is hardly necessary; since March 1988 numerous press releases have announced ozone depletion to be "worse than expected" [from the theory]--thus effectively discrediting it.
My comments have pointed to the lack--so far--of convincing observational evidence for long-term ozone depletion:
The data from ground-based observing stations are reported to be contaminated by UV absorption from atmospheric sulfur dioxide (1).
The statistical treatment is inadequate, with the derived "trend" strongly dependent on the time interval selected for analysis (2).
There is also the problem of disentangling any CFC effects from long-term ozone trends of natural origin, correlated with well-recorded trends in sunspot numbers (3).
Obviously, one cannot exclude the possibility of a long-term depletion of ozone due to anthropogenic causes, and specifically due to CFCs. But with each cause producing its characteristic "finger prints," proof must rely on a longer time series of more detailed observations (of CFC-specific altitude, latitude, and seasonal dependence). 2. While skeptical about the evidence for depletion, I consider the Antarctic ozone "hole" to be a genuine phenomenon, but have held a somewhat different view about its future. I have speculated (4) that--once there is sufficient chlorine present--the intensity of the hole is mainly controlled by the presence of polar stratospheric clouds (PSCs), and therefore by temperature and humidity rather than just atmospheric CFC concentration. Because the ongoing increase in atmospheric CO2 should gradually lower stratospheric temperatures (as a result of increased radiation loss), and the increase in methane should gradually increase stratospheric water vapor content (5), it is possible that the hole will persist--even if the chlorine concentration falls below the pre-1975 value. We don't know for certain where the chlorine threshold lies; it is possible therefore that an ozone hole could form in the Arctic if climate conditions favor the formation of PSCs there--even in the absence of CFC-produced chlorine (4).
3. Another controversial issue is covered by Taubes and in an adjacent article (6): What are the relative contribu- tions of natural and human sources to stratospheric chlorine? One side claims that the major sources are volcanic (7,8). The other side criticizes these estimates, arguing that nearly all of the chlorine emitted by volcanic and oceanic sources is washed out in the lower atmosphere, "with negligible quantities reaching the stratosphere" (9). A recent paper (10) claims removal of "up to" four orders of magnitude; but Taubes relates that El Chichon increased global stratospheric chlorine by 10 percent. I conclude that reliable statements about the relative effects of natural and human sources should be based on observed trends of stratospheric chlorine rather than on speculative calculations.
Rowland correctly quotes my views on sources of chlorine as of 1988 (11), but does not cite the relevant 1987 papers by Zander et al (12). They found that the total columns of HCl and HF (the major stratospheric reservoir gases for chlorine and fluorine) increased, from 1977 to 1986, at rates of (0.75 + 0.2)% and (8.5 + 1)% per year, respectively. Since HF is ascribed entirely to CFCs, the much lower trend for HCl would lead one to believe that there are large natural sources of stratospheric chlorine that overwhelm the CFC contribution.
This situation changed in 1991, however, when Curtis Rinsland et al, repeating Zander's measurements of solar IR spectra, reported increases for HCl and HF of (5.1 + 0.7)% and (10.9 + 1.1)% per year, respectively, for the period 1977-1990, thus suggesting CFCs as a major source (13). Nevertheless, Rinsland et al conclude--and I tend to agree: "...in contrast to HF, there are significant natural as well as anthropogenic sources of HCl."
According to Taubes, Rowland and others tag their opponents with "selective use of ...scientific papers and an equally discretionary choice of scientific results..." But in his "President's Lecture" Rowland quotes only papers that support his own view on CFC sources; the 1983 paper (14) he cites is in apparent disagreement with Zander's 1987 findings, and has been effectively criticized by Prinn (15).
I note in passing that the Montreal Protocol was signed in November 1987, and that production limits on CFCs were tightened in the period 1987 to 1991, when published scientific data indicated that CFCs were not an important source of stratospheric chlo- rine.
S. Fred Singer Science & Environmental Policy Project --------------------------------------------------------------------------------
62
posted on
09/22/2003 7:52:39 AM PDT
by
saminfl
To: saminfl
Interesting quote you supply. Let's see what Singer says.
Obviously, one cannot exclude the possibility of a long-term depletion of ozone due to anthropogenic causes, and specifically due to CFCs. But with each cause producing its characteristic "finger prints," proof must rely on a longer time series of more detailed observations (of CFC-specific altitude, latitude, and seasonal dependence). 2. While skeptical about the evidence for depletion, I consider the Antarctic ozone "hole" to be a genuine phenomenon, but have held a somewhat different view about its future. I have speculated (4) that--once there is sufficient chlorine present--the intensity of the hole is mainly controlled by the presence of polar stratospheric clouds (PSCs), and therefore by temperature and humidity rather than just atmospheric CFC concentration. Because the ongoing increase in atmospheric CO2 should gradually lower stratospheric temperatures (as a result of increased radiation loss), and the increase in methane should gradually increase stratospheric water vapor content (5), it is possible that the hole will persist--even if the chlorine concentration falls below the pre-1975 value. We don't know for certain where the chlorine threshold lies; it is possible therefore that an ozone hole could form in the Arctic if climate conditions favor the formation of PSCs there--even in the absence of CFC-produced chlorine (4).
In essence, this paragraph admits that Cl on polar stratospheric clouds causes ozone depletion in the Antarctic ozone hole. It also indicates that CFCs are a source of chlorine to the stratosphere.
Singer is a well-known skeptic on this; his stance on ozone depletion marginalized him even before he pushed his global warming skepticism with a petition hoax. While realizing that stating that runs the risk of being accused of an ad hominem argument, the remainder of what you supplied is clearly a skeptical argument, which is why I pointed this out.
It is absolutely true that low-level sources of HCl and HF "wash out" of the lower atmosphere and don't reach the stratosphere. If El Chichon increased stratospheric Cl by 10%, it was one of only two post-1970 eruptions powerful enough for major emissions to reach the stratosphere (the other being Pinatubo in 1991). Surely Singer doesn't think that one or two volcanoes are the major source of supply of Cl to the stratosphere. Or does he? This type of "uncertainty" argument is a typical skeptical way to argue.
Next question: does "total column" HCl and HF refer to tropospheric, mesospheric, and stratospheric concentrations, or just to stratospheric concentrations? That's not clear.
But the next paragraph is the most remarkable of all:
This situation changed in 1991, however, when Curtis Rinsland et al, repeating Zander's measurements of solar IR spectra, reported increases for HCl and HF of (5.1 + 0.7)% and (10.9 + 1.1)% per year, respectively, for the period 1977-1990, thus suggesting CFCs as a major source (13). Nevertheless, Rinsland et al conclude--and I tend to agree: "...in contrast to HF, there are significant natural as well as anthropogenic sources of HCl."
So Singer admits that CFCs are a major source of atmospheric HCl, but there are also natural sources. Well, there are, but this doesn't address or answer what the major sources of stratospheric Cl are. Is this a version of skeptical "bait and switch"? I can't tell without some context.
It appears that Singer's main line of argument is that CFCs are not a major source of stratospheric chlorine. Well, I posted this figure on another thread a few weeks ago; it shows stratospheric concentrations of CFCs as measured by an instrument (CLAES) on NASA's Upper Atmosphere Research Satellite (UARS):
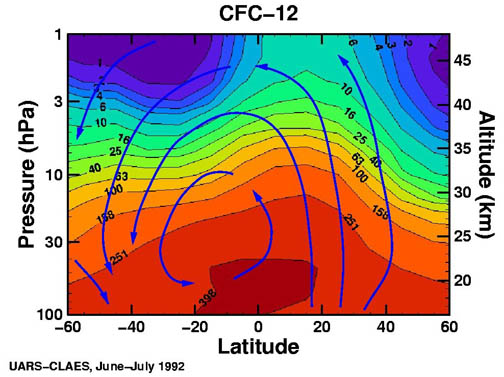
The units are parts per trillion by volume (pptv).
The source of this figure is a massive and comprehensive resource entitled Stratospheric Ozone: An Electronic Textbook. Virtually any question about the subject of stratospheric ozone and ozone depletion can be answered with this resource, if you're willing to take the time to read it.
Regarding Singer's final point, I'd like to know what reference he's referring to, and how he would assess this figure:
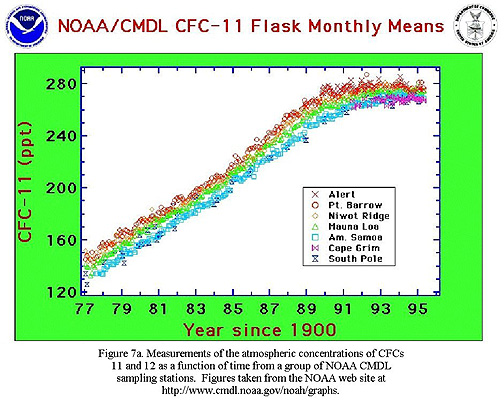
To: cogitator
http://www.sepp.org/ozone/ozdeplth.html
I thought I knew how to post a url, but I guess I was wrong.
64
posted on
09/22/2003 9:52:21 AM PDT
by
saminfl
To: cogitator
I'll keep that in mind the next time I plan a vacation.
To: Cicero
Look up F. Sherwood Rowland and his buddy, Mario Molina; they have gotten quite rich since their initial clamorings brought all this on.
I will say that industry has adjusted quite well to the changeover to alternative refrigerants and I suppose there is no reason why even scientists shouldn't engage in a bit of self-promotion now and then.
To: cogitator
One of the researchers who was at JPL in 1992 told me on the phone that the hole would grow larger until one day all the ozone would be gone from the upper atmosphere; he said it was too late to do anything about it.
I wish I could remember his name.
To: cogitator
It's the fault of France. The run all those millions of HDI diesels on the road. They're not allowed here because of high ozone emissions.
To: cogitator
The Argentine air force's Global Atmospheric Monitoring Station reported that during the two days of extreme risk in Tierra del Fuego, the Dobson units measurement, which indicates the thickness of the ozone layer, descended from the normal level of 300 to less than 200. The above excerpt is from http://www.spacedaily.com/news/ozone-02j.html
To: cogitator
Cleaning was done with R19 stable to 88F as a liquid, and R113 stable to 118F.
These two refrigerants were covered with a thin film of water to prevent evaporation and presented minimal flux losses.
To: WiscYooper
That was Mr. Dobson, himself.
To: ArrogantBustard
Sinusoidal, it is.
To: cogitator
The theory worked out by Molina and verified by Rowland does not allow for a low-limit to destruction as the effect is catalytic and there is no assumed loss of the catalyst proffered; I have boxes of studies and research on this subject.
Not one human death has yet been blamed or can be blamed on the loss of atmospheric ozone.
For every four molecules of oxygen, the sun can ionize three ozone molecules; since there are roughly 210,000PPM of O2 in the atmosphere a slice of 350PPM gives an efficiency rating of about .00167 - this implies that there is an upper-limit to the creation of ozone and allows for the constant breaking down of the unstable molecule.
For those couple of days when the ozone levels are expected or known to be low maybe the local cable system could rerun some Father Knows Best shows.
To: cogitator
So far, all the data show clearly that all the children play in the same sandbox; what is not clear is whether the wholesale elimination of the use and production of CFCs have directly influenced the readings of the last five years.
The shocking thing about this year's quite low readings is that it comes as the CFCs are diminishing at an ever rapid rate.
If the Cl released from these agents is indeed catalytic, then nothing we now do will stop the inevitable decline.
The very first molecule released back in 1939 may be the one now responsible for our concern; wouldn't that just bite your butt!?
To: saminfl
Here's how: start the line with a left caret, the caret equivalent of an open parenthesis, "(". Also known as a "less than" sign. Then type "A HREF="url", right caret, a text name for the URL, followed by left caret, "/A", right caret.
I copied the reference and took a look. The HF and HCl measurements are above mountaintops, but certainly not just stratospheric concentrations. That answers one question I had, and it indicates that the trend in atmospheric Cl concentrations may not be the same as the trend in stratospheric Cl concentrations.
To: Old Professer
One of the researchers who was at JPL in 1992 told me on the phone that the hole would grow larger until one day all the ozone would be gone from the upper atmosphere; he said it was too late to do anything about it.Extrapolation should only be performed under proper adult supervision and with all of the necessary safety equipment installed and operating. Otherwise you might get burned.
To: Old Professer
These two refrigerants were covered with a thin film of water to prevent evaporation and presented minimal flux losses.So I would presume that one of the major industrial sources was foam-blowing? (Not to be confused with the more common public-relations activity known as smoke-blowing.)
To: Old Professer
Not one human death has yet been blamed or can be blamed on the loss of atmospheric ozone.And let's be quite clear to any reading this reply that I never claimed or even pretended to claim that there had been any. (And with a proper nod to the dangers of spurious correlation, it should also be noted that sun-happy places like Australia have experienced significant increases in the rate of skin cancer. There's an ongoing public relations campaign in Australia to make the people practice solar exposure safety for just this reason.)
To: Old Professer
The shocking thing about this year's quite low readings is that it comes as the CFCs are diminishing at an ever rapid rate.But there's a reservoir effect; the Cl in the stratosphere will remain there for some time, and the CFCs in the stratosphere will keep breaking down to provide more Cl. So the recovery will take time. (Where have I heard THAT line before? Nevermind...)
If the Cl released from these agents is indeed catalytic, then nothing we now do will stop the inevitable decline.
It's only catalytic on the polar ice cloud particles. (Fortunately.) And the Cl concentration will decrease over time, so the CFC phaseout will have an effect eventually.
To: gatex
What are the concentrations of ozone-destroying chemicals as a function of time ?I've got more graphs if you want them. Scan up the discussion to the graphics I already posted; the "Stratospheric Ozone: An Electronic Textbook" link has more.
Navigation: use the links below to view more comments.
first previous 1-20, 21-40, 41-60, 61-80, 81-82 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson

