Posted on 07/04/2016 7:41:30 PM PDT by Jack Hydrazine
As far as I know there aren’t any gas stations for it to visit where we have to buy more fuel. It’s already been paid for, we can’t sell it to anybody else, and all we can do now is watch it.
It isn’t supposed to land, it is supposed to begin orbiting Jupiter, best I can tell.
*ping*
Don’t Juno it?
LOL!!!!
Wouldn’t it be more like a dunk than a crash?
But that’s what keeps it squeaky clean.
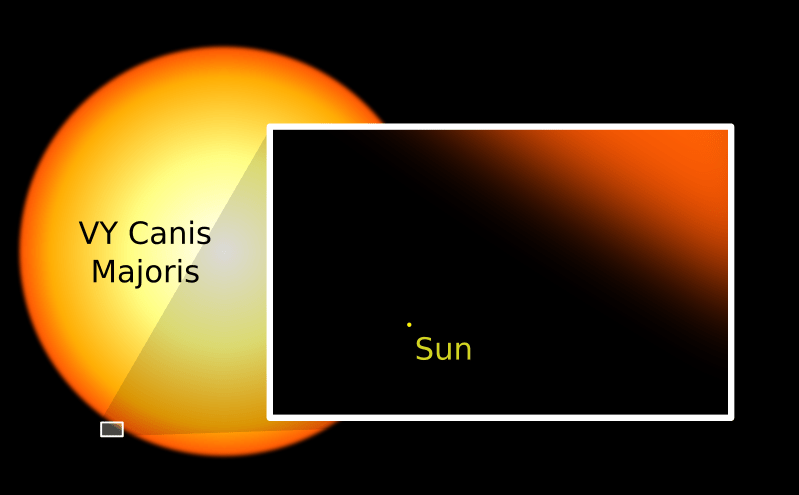
I saw an amazing website a year or so ago that had comparisons of planets in our solar system and beyond. [Should have kept the link, but didn’t.]
It went from small to larger to even larger. Compared to the largest object, Earth was not even the size of a grain of sand.
It was rather intimidating to see the pix and see how tiny Earth and its inhabitants are in the overall scheme of things.
Drops of Jupiter
by Train
https://www.youtube.com/watch?v=DbWcjdGmbzE
with lyrics.
https://www.youtube.com/watch?v=DbWcjdGmbzE
Hydrogen actually might be metallic near the core.
That would be an exotic kind of rock. I guess Juno doesn’t have something it can let out on a string down into that stuff and then reel it back in.
In her hair... told ya not to use Mr. Clean!
Great work! Juno is now in orbit!
To Scale: The Solar System
https://www.youtube.com/watch?v=zR3Igc3Rhfg
This was done out at the Black Rock Desert in northwestern NV, IIRC. This is where they have Burning Man every year as well as a couple of high power rocket launches where they can fly up to 200,000 feet.
Jupiter’s mass is too small for hydrogen to fuse to helium by initiating the triple alpha process nuclear fusion - creating a star.
So we would have the density needed to crunch hydrogen into a metallic condition, but not that needed to start a star.
Metallic hydrogen.
https://en.wikipedia.org/wiki/Metallic_hydrogen
Metallic hydrogen is a kind of degenerate matter, a phase of hydrogen in which it behaves as an electrical conductor. This phase was predicted theoretically in 1935.[2] It has yet to be unambiguously observed, but some observations consistent with metallic behaviour have been reported such as the possible observation of some new phases of solid hydrogen under static conditions,[3][4] and, in dense liquid deuterium, electrical insulator-to-conductor transitions associated with an increase in optical reflectivity.[5] At high pressure, on the order of hundreds of gigapascals, hydrogen might exist as a liquid rather than a gas. Liquid metallic hydrogen is thought to be present in large amounts in the gravitationally compressed interiors of Jupiter, Saturn, and in some extrasolar planets.
Though at the top of the alkali metal column in the periodic table, hydrogen is not, under ordinary conditions, an alkali metal. In 1935 physicists Eugene Wigner and Hillard Bell Huntington predicted that under an immense pressure of around 25 GPa (250000 atm or 3500000 psi), hydrogen atoms would display metallic properties, losing hold over their electrons.[2] Since then, metallic hydrogen has been described as “the holy grail of high-pressure physics”.[6]
The initial prediction about the amount of pressure needed was eventually proven to be too low.[7] Since the first work by Wigner and Huntington, the more modern theoretical calculations were pointing toward higher but nonetheless potentially accessible metallization pressures. Techniques are being developed for creating pressures of up to 500 GPa, higher than the pressure at the center of the Earth, in hopes of creating metallic hydrogen.[8]
In March 1996, a group of scientists at Lawrence Livermore National Laboratory reported that they had serendipitously produced, for about a microsecond at temperatures of thousands of kelvins, pressures of over a million atmospheres (>100 GPa) and density of approximately 0.6 g/cm3,[16] the first identifiably metallic hydrogen.[17] The team did not expect to produce metallic hydrogen, as it was not using solid hydrogen, thought to be necessary, and was working at temperatures above those specified by metallization theory. Previous studies in which solid hydrogen was compressed inside diamond anvils to pressures of up to 2,500,000 atm (250 GPa), did not confirm detectable metallization. The team had sought simply to measure the less extreme electrical conductivity changes which were expected to occur. The researchers used a 1960s-era light-gas gun, originally employed in guided missile studies, to shoot an impactor plate into a sealed container containing a half-millimeter thick sample of liquid hydrogen. The liquid hydrogen was in contact with wires leading to a device measuring electrical resistance. The scientists found that, as pressure rose to 1,400,000 atm (140 GPa), the electronic energy band gap, a measure of electrical resistance, fell to almost zero. The band-gap of hydrogen in its uncompressed state is about 15 eV, making it an insulator but, as the pressure increases significantly, the band-gap gradually fell to 0.3 eV. Because the thermal energy of the fluid (the temperature became about 3000 K due to compression of the sample) was above 0.3 eV, the hydrogen might be considered metallic.
In 2011 Eremets and Troyan reported observing the liquid metallic state of hydrogen and deuterium at static pressures of 260–300 GPa.[3] This claim was questioned by other researchers in 2012.[25][26]

I don’t think you understand the gravity of the situation.
I’m still too upset over Harembe’s murder to think about stuff like this.
He was a good—no, great ape.
And how much does this project cost? another few billion to siphon from the public? ridiculous.....
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.