I thought your question was regarding the Second Law and that your complaint was that light would inevitably degrade molecules. Why don't we stick with that for now?
Light is used by chemists to catalyze a wide variety of reactions that are thermally forbidden, and yes, most of these are irreversible. Previously these reactions used to be done just by setting the reaction flask in the windowsill or on the benchtop. Now we have apparatuses that produce the optimal frequences, but the principle is the same. Here is an example:
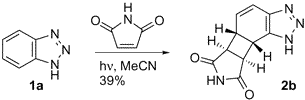
This is a nice example because it is an intermolecular reaction resulting in a product that is very highly functionalized.
"Photochemistry of Benzotriazole: An Unprecedented Tautomer-Selective Intermolecular [2+2] Photocycloaddition." Booker-Milburn, K.; Wood, P.; Dainty, R.; Urquhart, M.; White, A.; Lyon, H.; Charmant, J. Organic Letters, 2002, 4, 1487-1489.
Obviously then light can initiate such reactions and objections on Second Law grounds are not in keeping with reality.
Nice reaction. Where did the benzotriazole come from?
Plus
The two relevant reactants were dissolved in acetonitrile, and transferred into a Pyrex photo reactor immersion well. The solution was degassed for 10-15 minutes by the bubbling of nitrogen through the solution. Irradiation of the solution under a nitrogen atmosphere for the specified length of time was achieved by the use of a 125 W medium pressure mercury discharge lamp. After the reaction was complete, the solvent was removed in vacuo and the resultant crude photoadduct was purified by flash column chromatography using the solvent system stated.
Why do you suppose they degassed the solution?
Finally, for one experiment..
A solution of benzotriazole (2.68 g, 22.5 mmol) and maleimide (0.43 g, 4.43 mmol) in CH3CN (100 ml) was irradiated according to the general photolysis procedure for 16 h. After this time an insoluble brown precipitate had formed, this was collected by suction filtration and washed with EtOAc (2 × 50 ml) to give 2a as a brown solid (0.37 g, 39%; mp 289-290 °C);
2.68 + .43 - .37 = 2.74
Where do you suppose that 2.74 grams went?
