Posted on 12/05/2009 10:40:14 PM PST by neverdem
French scientists have demonstrated the potential of a new fuel cell catalyst inspired by hydrogenase enzymes. Although its activity doesn't yet match that of platinum, the researchers say it is the first useful biomimetic catalyst capable of operating under fuel cell conditions.
In a hydrogen economy, power would be generated by oxidising stored hydrogen in fuel cells. This reversible reaction - the opposite of which produces hydrogen through the electrolysis of water - can be driven by platinum-based catalysts. Nature, however, in hydrogenase enzymes, has evolved a way of doing this without the need for such rare metals and thus borrowing from nature's design may be a way to create fuel cell catalysts on the cheap.
Vincent Artero at Joseph Fourier University and colleagues have achieved a significant step towards this goal with a nickel bisdiphosphine-based design. Artero explains that it is the first time this has been shown for such a sophisticated bio-inspired compound. 'Most people think these compounds are nice achievements of academic research, but that they will never be stable under technological conditions - under pressure, under heat and in very acidic solutions,' he says. 'We have demonstrated that we can use these compounds under the conditions that are used in the fuel cells, or electrolysers, that are developed at the moment.'

|
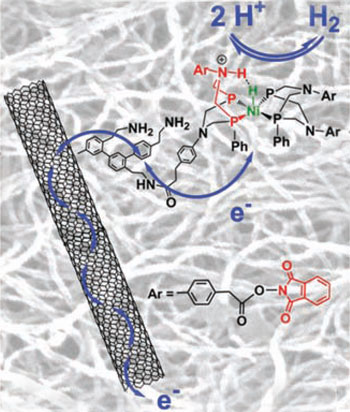
Structure of the bio-inspired hydrogen-evolving nickel catalyst grafted on a carbon nanotube
© Science
|
A close look at the catalyst reveals a striking similarity to the metalloproteins on which it is modelled. At the centre is a nickel atom, as in nickel-iron hydrogenases, combined with a diphosphine ligand bearing a basic N-H that mimics a co-factor in iron-iron hydrogenases and helps to control proton movement as hydrogen is either produced or oxidised. Artero's team grafted their complexes onto electrically conducting carbon nanotubes that drive electrons to or from the active site and embedded them in a polymer to protect them from acidic electrolytes - mimicking the protection afforded by polypeptide chains in enzymes. The result is a catalyst that shows impressive efficiency and stability under operating conditions.
Dan DuBois, at the Institute for Interfacial Catalysis in Richland, Washington State, developed the original design on which Artero's catalyst is built. 'They've really shown that you can take these catalysts and put them in an operating cell,' he says. 'They're actually functioning quite nicely, and I think that if we can get another one or two orders of magnitude in rate, which is doable, then they will be useful.'
Artero explains that the rate difference may be due to bulky functional groups used to anchor the catalysts to the nanotubes. 'We think that may have slowed the catalytic activity,' he says. 'So we can now try to imagine another way to graft the catalyst and to keep the catalytic activity high.' But he also points out that, crucially, the overvoltage - the amount of energy 'wasted' to drive the reaction at a sufficiently high rate - does approach that of platinum, unlike most other non-Nobel metal designs.
bump!
Interesting!
If they keep this up, the machines may not have to enslave us for use as a power source! :p
The problem with bio based alternatives is like food, overtime it will fall apart (ie rot). To prevent that, the scientist must come up with some additive that will keep the cheap bio alternative stable over long periods of time. Unfortunately most of the additives are exotic and expensive to fabricate. It will neutralize the low cost advantages of bio based alternatives. Example, scientists have fabricated bio solar panels that uses photosynthesis to generate energy like plant leaves. Problem is the solar panels will rot unless it is feed neutrients like a plant which negates its low material cost advantages. Additives were created to preserve the bio solar panel, but they turn out to be extremely expensive and exotic to produce. Net effect is bio type solar panels became too expensive in practice compared to silicate based systems.
Then they wont have a need for us and we will be eliminated.
Seems to me the methane economy should come before the hydrogen economy. and before that should come the urea/amonia/acetylene/peroxide/propane/LPG economy.
Fouorty five years ago, I drove a mail truck past Varian Aerograph 6 days a week. It was impossible to not notice their readerboard sign that promised "imminent break-throughs in solar cell efficency" that would change the world. There were also regular articles and interviews in the local press beating the same drum.
I'm still waiting, but Varian isn't; they aren't, so far as I can tell, even still in that business---but other keep telling me that any year now, THEY will succeed where Varian failed, and make a truly efficient, cheap, world changing solar cell.
Some decades ago, I also began hearing the same noise about fuel cells.
Several years after that, it was 'turkey guts into oil'.
Some things never change...coal, oil, and nukes WORK, and have for anywhere from 60+ to 200+ years...and so have snake oil salesmen and starry eyed "could, should, maybe, someday" researches in search of grants or patronage.
The only thing I see that shows any hope for the next 50 years is batteries. Rechargeable batteries have come a long long way in the last 25 years.
bump
RNA Silencer Shows Promise for Hepatitis C
Autism and schizophrenia could be genetic opposites
Knowing What’s Worth Paying for in Vitamins
FReepmail me if you want on or off my health and science ping list.
ping
BTTT


Awhile ago I read that someone had created a way to increase lithium-ion battery capacity by 10. Another article was about someone who had devised a way to fully recharge a lithium-ion battery in 10 minutes.
Where are the products?!?!
You are referring to one of two new developments in battery tech, I beleive:
1. lithium ion air batteries
2. Li ion nanowire batteries
The first is a long ways away I think. I dont know much but I think they figured out a way to replace the electrolyte with air. Somehow the battery sucks O2 from the air(atmosphere) to produce electric potential.
The second is about 5 years away and they have now downgraded the performance increase to something less than 5 times current battery capacities.
The fast recharge batteries were developed by sanyo, I think. Those should be on the market for cordless powertools and RC modellers any day now. I don’t know what they are called(the name of them). They might even already be here. I don’t own any cordless power tools or RC models so I wouldn’t know.
French scientists. Thanks neverdem.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.