|
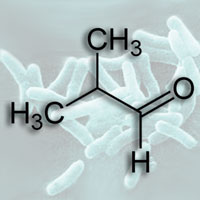
Liao and his team used genetically modified cyanobacteria to produce isobutyraldehyde from carbon dioxide
|
Posted on 11/15/2009 6:10:01 PM PST by neverdem
US researchers have genetically modified bacteria to eat carbon dioxide and produce isobutyraldehyde - a precursor to several useful chemicals, including isobutanol, which has great potential as a fuel alternative to petrol.
The modified bacteria are highly efficient and powered by sunlight, so a future goal is to set up colonies near to industrial plants. This would allow greenhouse gases to be recycled into useful chemical feedstock - supplying several hydrocarbons that are typically obtained from petroleum.

|
Liao and his team used genetically modified cyanobacteria to produce isobutyraldehyde from carbon dioxide
|
'Here, we were successful in engineering CO2-eating bacteria to produce isobutyraldehyde very efficiently,' says James Liao, who led the work at the University of California, Los Angeles, US. 'Our process is around 10 times faster than hydrogen production and about 100 times faster than genetically engineered ethanol production.'
Liao's team modified the genome of the cyanobacteria Synechococcus elongatus by incorporating four genes from other bacteria into the structure. These genes, which come from L. lactis, B. subtilis and E. coli, produce enzymes that hijack the metabolism of the microbes - turning them into miniature reaction vessels.
The synthetic pathway begins with the photosynthetic conversion of CO2 to pyruvic acid by the bacteria. Next, the reaction continues in the bacteria as the added genes trigger three further steps to make isobutyraldehyde.
Importantly, extracting the final product from the mix is a simple process. 'The fuel vaporises to the gas phase easily, making separation extremely simple. Afterwards, we can liquefy it again by simple condensation,' Liao told Chemistry World.
This process helps keep the bacteria alive longer, as they are not exposed to large amounts of chemicals. 'The bacteria are very stable, and in our flasks without much environmental control, the bacteria continued to produce for about 10 days,' Liao says.
But there are still much work to be done before the process can be commercialised, Liao notes, particular with scaling up the process.
micro ping
Now, if they can make one that eats BullS**t and turns it into water, Al Gore would be gone, the desert would bloom, and we’d be all set!
A nice piece of work if they can make it economically practical, but be forewarned - isobutyraldehyde has a pungent odor, is an irritant to the eyes and lungs, and with oxidation is readily contaminated by isobutyric acid, which smells pretty much like essence of vomit.
Reducing it down to isobutyl alcohol, if economically viable, would give a more energy rich fuel from which the nasty properties laid out above would by largely absent.
But, but shouldn’t we be getting rid of carbon dioxide? The bacteria won’t have anything to eat.
yes but why bother when algae can be made to oil from their lipids.
I'm suspectin' the scientists who discovered how to copy the process knew all about this!
Bet their co-workers in the field of "Global Warming" weren't told either ~ worth a note, while looking up the URL for that one I ran across a statement to the effect that if we can get atmospheric oxygen down to 200 ppm all the plants will die, and so will we, in an almost permanent situation.
Oh no!
If these things get loose in the wild and reproduce, WE’RE ALL GONNA DIE! they’ll eat up all the CO2, the plants will die, and then the oxygen will dissappear too!
Its the end of the world!
Thanks for the link.
I assume you meant CO2, not oxygen. Then we'll have the next ice age! /s
I think obligate aerobic critters like us would be SOL with atmospheric oxygen down to 200 ppm.
The sooner these global warming alarmists are thoroughly discredited, the better. The sooner we have cheap energy, the better. Thanks for the URL.
The GW co-workers would have tried to get the grants pulled these researchers were working under. After all a natural/nearlynatural process that can be scaled up to convert significant quantities of CO2 into "stuff" and oxygen will destroy the very basis of the GW argument.
This also means GE is a gonner, as are all the guys peddling windpower. Ramping up pond scum production is one of those things that can be done so readily ~
Let’s count the picoseconds before the Democrats try to ban this as environmentally unsafe.
bump
Thanks!
I hear there are these things called “plants”, and they turn CO2 into all sorts of useful things — food, fuel, building materials, recreational drugs, medicine...
Only problem is, their waste product is a flammable, corrosive gas called “oxygen”. It’s polluting our air and, when combined with hydrogen, creates a deadly liquid.
Butanol DOES work as a transportation fuel, although with some significant differences from what it might replace. It has little affinity for water, but can be mixed with gasoline in almost any proportion with very little loss of energy density by volume, and as an oxygenating agent it is nearly as effective as ethanol but without the contamination problems that make shipping and handling ethanol difficult.
However, I don’t know that this pathway is the best choice alternative to T-fuel made from crude oil, natural gas, coal, or biomass. All of the crude oil that we know how to find, extract, refine, and distribute came from pre- historic ALGAE beds that were buried, processed by subterranean heat and pressure into KEROGEN, and extracted from wells for our use.
I suspect that we will find ways to shortcut this process using bio-engineered algae, sunlight, brackish water, and waste carbon dioxide to produce “green crude,” which can be fed directly into our existing refineries and processed into the same products we now use. This is an alternative worthy of investigation and development, but I still think Algae will win.
Interesting...
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.