Skip to comments.
Big Corn and Ethanol Hoax
creators.com ^
| March 12, 2008
| Walter E. Williams
Posted on 04/28/2008 9:53:50 PM PDT by paltz
One of the many mandates of the Energy Policy Act of 2005 calls for oil companies to increase the amount of ethanol mixed with gasoline. President Bush said, during his 2006 State of the Union address, "America is addicted to oil, which is often imported from unstable parts of the world." Let's look at some of the "wonders" of ethanol as a replacement for gasoline.
Ethanol contains water that distillation cannot remove. As such, it can cause major damage to automobile engines not specifically designed to burn ethanol. The water content of ethanol also risks pipeline corrosion and thus must be shipped by truck, rail car or barge. These shipping methods are far more expensive than pipelines.
Ethanol is 20 to 30 percent less efficient than gasoline, making it more expensive per highway mile. It takes 450 pounds of corn to produce the ethanol to fill one SUV tank. That's enough corn to feed one person for a year. Plus, it takes more than one gallon of fossil fuel — oil and natural gas — to produce one gallon of ethanol. After all, corn must be grown, fertilized, harvested and trucked to ethanol producers — all of which are fuel-using activities. And, it takes 1,700 gallons of water to produce one gallon of ethanol. On top of all this, if our total annual corn output were put to ethanol production, it would reduce gasoline consumption by 10 or 12 percent.
Ethanol is so costly that it wouldn't make it in a free market. That's why Congress has enacted major ethanol subsidies, about $1.05 to $1.38 a gallon, which is no less than a tax on consumers. In fact, there's a double tax — one in the form of ethanol subsidies and another in the form of handouts to corn farmers to the tune of $9.5 billion in 2005 alone.
There's something else wrong with this picture. If Congress and President Bush say we need less reliance on oil and greater use of renewable fuels, then why would Congress impose a stiff tariff, 54 cents a gallon, on ethanol from Brazil? Brazilian ethanol, by the way, is produced from sugar cane and is far more energy efficient, cleaner and cheaper to produce.
Ethanol production has driven up the prices of corn-fed livestock, such as beef, chicken and dairy products, and products made from corn, such as cereals.
As a result of higher demand for corn, other grain prices, such as soybean and wheat, have risen dramatically. The fact that the U.S. is the world's largest grain producer and exporter means that the ethanol-induced higher grain prices will have a worldwide impact on food prices.
It's easy to understand how the public, looking for cheaper gasoline, can be taken in by the call for increased ethanol usage. But politicians, corn farmers and ethanol producers know they are running a cruel hoax on the American consumer. They are in it for the money. The top leader in the ethanol hoax is Archer Daniels Midland (ADM), the country's largest producer of ethanol. Ethanol producers and the farm lobby have pressured farm state congressmen into believing that it would be political suicide if they didn't support subsidized ethanol production. That's the stick. Campaign contributions play the role of the carrot.
The ethanol hoax is a good example of a problem economists refer to as narrow, well-defined benefits versus widely dispersed costs. It pays the ethanol lobby to organize and collect money to grease the palms of politicians willing to do their bidding because there's a large benefit for them — higher wages and profits. The millions of gasoline consumers, who fund the benefits through higher fuel and food prices, as well as taxes, are relatively uninformed and have little clout. After all, who do you think a politician will invite into his congressional or White House office to have a heart-to-heart — you or an Archer Daniels Midlands executive?
Walter E. Williams is a professor of economics at George Mason University. To find out more about Walter E. Williams and read features by other Creators Syndicate writers and cartoonists, visit the Creators Syndicate Web page at www.creators.com.
COPYRIGHT 2008 CREATORS SYNDICATE, INC.
TOPICS: Business/Economy; Government; News/Current Events
KEYWORDS: carboncult; energy; ethanol; walterewilliams; walterwilliams
Navigation: use the links below to view more comments.
first previous 1-20 ... 81-100, 101-120, 121-140, 141-159 next last
To: DoughtyOne
Until that new device was invented, you could have come to me and said, desalinizing water will never be competitive, that’s just the reality of the physics involved. You're a reasonable guy, so its worth explaining in detail.
What you're describing is a breakthrough in desalinization processes, not a re-writing of the 1st and 2nd Laws of Thermodynamics.
Hydrogen "burning" is fundamentally constrained since the only large source of hydrogen we have is water. So, to get hydrogen "fuel," you have to break its bond with oxygen, which requires energy. Great, now we have our fuel. You put it in your tank, your engine mixes it with air, compresses it, and ignites it so that it reacts with the oxygen in the air. What do you have now?
Water. The same molecule you started with.
The energy balance is constrained by the 1st and 2nd Law of Thermo. It took you more energy to separate the hydrogen from the oxygen than you could ever produce by reacting it with oxygen. thackney is being kind by approximating the losses at 30%. It will be in reality a massive loss of energy, due to the inefficiencies of compressing the "fuel" to a useful volume and the horrendous thermodynamic efficiency of even the best internal combustion engines available today. Even the most advanced gas and steam combined-cycle turbine system available today is, at best, 60% efficient. Your car's engine is a wasteful pig in comparison.
These are fundamental laws, not open for debate. Trying to design a process that somehow circumvents these facts is a fool's errand.
Hydrogen = a battery. It would be far more efficient to simply use the electrical energy you started with to power electric cars.
To: GregoryFul
"So how come ethanol is not sent by pipeline?" Damned if I know. I suspect it's economics (no single production site large enough to justify a dedicated pipeline, nor close enough to an existing pipeline to be sent in "batches"). But the question is irrelevant. Williams is simply wrong about the chemical engineering of the ethanol process. If he can get something as basic as what the process produces (anhydrous ethanol as compared to 95%), then he's PROBABLY wrong about a lot other stuff.
To: Wonder Warthog; GregoryFul
"So how come ethanol is not sent by pipeline?"Corrosion. Ethanol cannot be produced 100% water free. Even "200 proof" has trace water in it. Someday maybe they'll come up with a lined pipe cost-effective enough for the task but for now it's a no go.
103
posted on
04/30/2008 7:09:30 AM PDT
by
facedown
(Armed in the Heartland)
To: facedown; GregoryFul
"Corrosion. Ethanol cannot be produced 100% water free. Even "200 proof" has trace water in it. Someday maybe they'll come up with a lined pipe cost-effective enough for the task but for now it's a no go." Wrong. I guarantee you that ethanol passed over mole sieves will be water free. It may not STAY water-free, but it will damned well start out that way. And the corrosion of pipelines is irrelevant to the use of ethanol blends in automobiles, because todays cars are built to handle it. Of course, you shouldn't use it in your 1940's classic Lincoln V12, or your 1960's 'Vette (but then, you shouldn't use unleaded gas in them either) but anything manufactured after about 1980 should be fine.
To: Wonder Warthog; GregoryFul
I guarantee you that ethanol passed over mole sieves will be water free.No kidding, we're talking about real-world economics.
It may not STAY water-free, but it will damned well start out that way.
Bingo.
And the corrosion of pipelines is irrelevant to the use of ethanol blends in automobiles,...
Not the subject at hand.
I was trying to explain the practical and cost reasons that ethanol is not transported by pipeline.
Sheesh.
105
posted on
04/30/2008 7:31:42 AM PDT
by
facedown
(Armed in the Heartland)
To: facedown; GregoryFul
"I was trying to explain the practical and cost reasons that ethanol is not transported by pipeline. Sheesh." Yes, but Gregoryful was trying to float that red herring (pipeline corrosion) as support for Williams blatant screwup about ethanol plants producing 95% ethanol and how it was "bad for cars" because of the water content.
I simply haven't studied the issue of pipeline corrosion by ethanol (I HAVE studied pipeline corrosion by hydrogen--another red herring argument used to denigrate the hydrogen economy idea). But I'll pull out my "Corrosion Engineer's Handbook" and give it a quick look.
To: thackney
I appreciate the point. Now, what happens if we find a way to separate the hydrogen in a manner that doesn't take more energy to achieve than we can obtain from the hydrogen after the separation?
I guess I'm not quite getting this, because electrolysis can be used to split the hydrogen away from the oxygen. Explain to me why using solar to do that would be cost prohibitive.
Is there another step in the process that causes you to say it costs too much?
107
posted on
04/30/2008 9:50:31 AM PDT
by
DoughtyOne
(McCain is a poison pill. Accept it! http://www.freerepublic.com/focus/f-news/2006492/posts)
To: Sunnyflorida
Yes, that is in many instances natural seepage.
I'm going to be a lot happier, when we're not exposed to the whims of the Muslim states or the likes of Hugo Chavez.
108
posted on
04/30/2008 9:57:09 AM PDT
by
DoughtyOne
(McCain is a poison pill. Accept it! http://www.freerepublic.com/focus/f-news/2006492/posts)
To: DoughtyOne
what happens if we find a way to separate the hydrogen in a manner that doesn't take more energy to achieve than we can obtain from the hydrogen after the separation? Then you have overturned the laws of physics and can condense the water after combustion of hydrogen, start the process over and build a perpetual motion machine.
Or you can stay in the present Universe as described previously.
Sorry for the sarcasm but this as basic as trying to use water in its natural occurring state as fuel for a fire.
Explain to me why using solar to do that would be cost prohibitive.
This isn't an item of cost. If you use solar to generate the electricity, use an electric car and batteries. There is less wasted energy even with using the existing batteries of today. Putting Hydrogen in the middle just consumes more energy with less output.
109
posted on
04/30/2008 10:00:44 AM PDT
by
thackney
(life is fragile, handle with prayer)
To: Wonder Warthog
Ethanol is terrible for boats.
The diversion is raising feed costs. And the taxpayers are paying for it. Now if a farmer on their own wants to make it and market it I have no problem. But to force it on people is just wrong.
110
posted on
04/30/2008 10:11:23 AM PDT
by
Sunnyflorida
(Drill in the Gulf of Mexico/Anwar & we can join OPEC!!! || Write in Thomas Sowell for President.)
To: DoughtyOne
“I’m going to be a lot happier, when we’re not exposed to the whims of the Muslim states or the likes of Hugo Chavez.”
I agree. But the key is to be a net exporter. No issue in importing here and their as long as we are net exporters. This is very achievable.
111
posted on
04/30/2008 10:12:49 AM PDT
by
Sunnyflorida
(Drill in the Gulf of Mexico/Anwar & we can join OPEC!!! || Write in Thomas Sowell for President.)
To: CPT Clay
Others have mentioned that. You folks are probably right. To a lay person it’s kindof a tough sell for folks to tell us that oil can be pumped out of the ground and placed in large tanks, then pumped into an oil tanker, then shipped half-way around the planet, off-loaded to a refinery, be processed through that refinery and still not be more costly than the energy we could get out of it.
On the other hand we have an ocean full of hydrogen that can be split off, but it’s too costly to pump it through a pipe and use electrolosis to do the deed.
I don’t see how it could cost more than $3.00 to split off a gallons worth of hydrogen, but that’s the case you folks are making. Still sounds strange to me.
Thanks for the comments.
112
posted on
04/30/2008 10:13:09 AM PDT
by
DoughtyOne
(McCain is a poison pill. Accept it! http://www.freerepublic.com/focus/f-news/2006492/posts)
To: DoughtyOne
I don’t see how it could cost more than $3.00 to split off a gallons worth of hydrogen Do you have any basis for that number other than you wish it were so?
113
posted on
04/30/2008 10:24:33 AM PDT
by
thackney
(life is fragile, handle with prayer)
To: DoughtyOne
Hydrogen production is not new or under-researched.
Oil companies are one of the largest users of hydrogen. It is used in the refineries for processes downstream of the distillation column like hydrotreaters.
Steam Reforming of Natural Gas is MUCH cheaper than electrolysis of water to produce hydrogen.
Hydrogen Production and Delivery
http://www.nrel.gov/hydrogen/proj_production_delivery.html
114
posted on
04/30/2008 10:30:54 AM PDT
by
thackney
(life is fragile, handle with prayer)
To: Palmetto
Perhaps I’m as dense as a stack of bricks, but I still do not understand how it cost more than $3.00 to make a gallon (a comparable measure to a gallon of gas) of hydrogen.
What does it cost ot make that gallon of hydrogen? Is it $10, $25, $50, still higher? If you were to tell me that it cost more than $1 worth of electricity, I’d be surprised, but not being a physicist or associated with that industry, I have no idea what it actually costs.
Give me some idea what it actually costs to make the gallon of hydrogen.
BTW, I am familiar with the process of splitting hydrogen out and it joining with the oxygen again upon burning. To me that is the beauty of it. You don’t have to mess with batteries.
115
posted on
04/30/2008 10:32:49 AM PDT
by
DoughtyOne
(McCain is a poison pill. Accept it! http://www.freerepublic.com/focus/f-news/2006492/posts)
To: thackney
Please tell me what it costs to make a gallon of hydrogen.
I said I didn’t see how it could cost more than $3.00 to produce a gallon of hydrogen. Your comeback should have been something like, “The cheapest method to date costs $13.87 to produce that gallon of hydrogen.”
What I am getting are vague comments about physics and laws of nature, but nothing relating to actual tangible economic numbers.
116
posted on
04/30/2008 10:40:58 AM PDT
by
DoughtyOne
(McCain is a poison pill. Accept it! http://www.freerepublic.com/focus/f-news/2006492/posts)
To: DoughtyOne
a kilogram of hydrogen contains about the same energy as a gallon of gasoline.
Source:
www.hydrogen.energy.gov/docs/cs_distr_steam_methane_reform.doc
The following graph compares to processes. A larger industrial sized facility and an smaller commercial sized facility. The electricity cost are the biggest factor. Industrial rates were $0.045/kWh and Commercial rates were $0.069/kWh for this study.
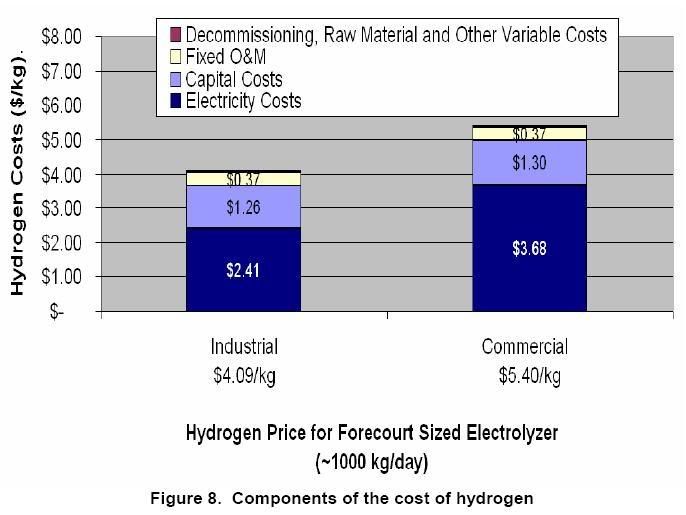
Source:
Electrolysis: Information and Opportunities for Electric Power Utilities
National Renewable Energy Laboratory
http://www.nrel.gov/hydrogen/pdfs/40605.pdf
This doesn't equate to price at the pump for gasoline, but price at the refinery. Now it needs to be transported, distributed and sold through the retail process. Add the government road taxes and sales taxes.
Then you could compare price to gasoline at your pump.
Now, those electricity prices are cheaper than reality. In 2006 the industrial average price was 6.16¢/kWH and the commercial was 9.46¢/kWH. Scale up the previous chart.
117
posted on
04/30/2008 10:55:00 AM PDT
by
thackney
(life is fragile, handle with prayer)
To: thackney
Assuming the smaller commercial sized unit is located in a major market near a place like LA, Houston or Chicago. Then it doesn't need a bunch of distribution cost but is sold only from the facility. Scaling up the electricity cost to 2006 prices hydrogen is $6.77 for an equivalent gallon of gasoline. Of course California has much higher electricity costs than Houston.
How much tax to add?
In Texas, the average is about 38¢ per gallon for gasoline.
California is 64¢. Although California uses a percent sales tax that would go up with the higher price.
http://www.gasbuddy.com/tax_info.aspx
So at least $7 a gallon in 2006. My electricity rates have gone up since then. I suspect most everyone elses has as well.
118
posted on
04/30/2008 11:05:23 AM PDT
by
thackney
(life is fragile, handle with prayer)
To: thackney
What happens if we find a way to separate the hydrogen in a manner that doesn't take more energy to achieve than we can obtain from the hydrogen after the separation?
Then you have overturned the laws of physics and can condense the water after combustion of hydrogen, start the process over and build a perpetual motion machine.
Okay, that' was poor wording on my part. I don't expect there to be a net increase of energy. I expect there to be a reasonable cost of conversion.
Or you can stay in the present Universe as described previously. Sorry for the sarcasm but this as basic as trying to use water in its natural occurring state as fuel for a fire.
That's okay, I didn't think the comment out and infered something I know is impossible.
Explain to me why using solar to do that would be cost prohibitive.
This isn't an item of cost. If you use solar to generate the electricity, use an electric car and batteries. There is less wasted energy even with using the existing batteries of today. Putting Hydrogen in the middle just consumes more energy with less output.
In an economic sense, it is always an item of cost. I understand that your preference is to go electric. I understand your reasoning behind it, and I know it makes sense energy unit per energy unit. There are certainly things that I like about the idea. I don't see packing cars full of batteries to be a great alternative to what we are doing today. My preference would be to used hydrogen to generate the electricity on board the car to drive an electric drive system. That would probably be a less efficient way to do things, but would it be cost prohibitive?
My goal is to replace our current system with one that is cost comparitive. If it currently costs me $0.37 cents a mile to get to my destination, what will it cost me to use hydrogen to get to the destination? Will that cost be $0.17 per mile or $2.18?
If you're telling me that it would cost $2.18 per mile and that is rock solid, then you have a point. If you're telling me I could go the distance at a cost of $0.27 per mile with batteries, and $0.32 per mile with hydrogen, I'd probably prefer the hydrogen.
119
posted on
04/30/2008 11:09:08 AM PDT
by
DoughtyOne
(McCain is a poison pill. Accept it! http://www.freerepublic.com/focus/f-news/2006492/posts)
To: Sunnyflorida
We have plenty of natural gas and oil to power cars. No, we don't. At least we don't have enough to meet the current demand, let alone any growth in the future.
120
posted on
04/30/2008 11:11:05 AM PDT
by
IronJack
(=)
Navigation: use the links below to view more comments.
first previous 1-20 ... 81-100, 101-120, 121-140, 141-159 next last
Disclaimer:
Opinions posted on Free Republic are those of the individual
posters and do not necessarily represent the opinion of Free Republic or its
management. All materials posted herein are protected by copyright law and the
exemption for fair use of copyrighted works.
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson
