To: thackney
Sorry, but you are quite mistaken. Ref:
fact, not friction.
You forgot that ANY gas can be liquefied. All you need is enough pressure OR a low enough temperature.
Or a suitable combination of both.
26 posted on
05/20/2015 11:50:26 AM PDT by
Don W
( When most riot, neighborhoods and cities burn. When Whites riot, nations and continents burn.)
To: Don W
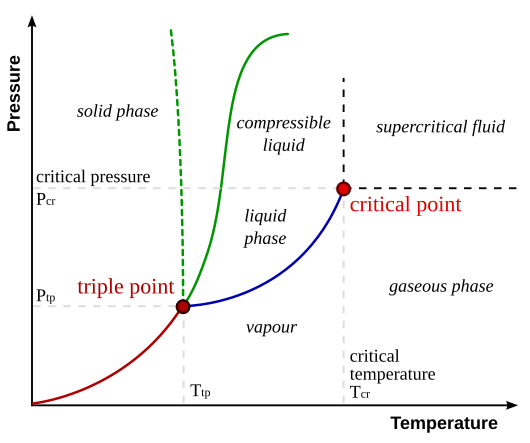
Sorry, you need to understand Critical Temperature.
http://www.chem.purdue.edu/gchelp/Liquids/critical.html
Gases can be converted to liquids by compressing the gas at a suitable temperature.
Gases become more difficult to liquefy as the temperature increases because the kinetic energies of the particles that make up the gas also increase.
The critical temperature of a substance is the temperature at and above which vapor of the substance cannot be liquefied, no matter how much pressure is applied.
27 posted on
05/20/2015 12:01:00 PM PDT by
thackney
(life is fragile, handle with prayer)
To: Don W
You may want to also notice the chart you linked stops at
190.53°Kelvin, which equals -116.7° Fahrenheit
Above that temperature, which is the critical temperature for methane, no liquid state exists, regardless of pressure.
28 posted on
05/20/2015 12:06:42 PM PDT by
thackney
(life is fragile, handle with prayer)
FreeRepublic.com is powered by software copyright 2000-2008 John Robinson
